Hypovolaemic shock
Dehydration without shock can generally be managed with oral rehydration fluid and solids. Children with shock (hypotension and acidosis) caused by hypovolaemia should be given parenteral fluid immediately: administer 20 mL/kg repeatedly until plasma volume is restored (20–100 mL/kg may be required). Fluid can be given i.v. or into the bone marrow (see p. 39); NG or intraperitoneal fluid is not effective in shock.
Use 0.9% saline to restore intravascular volume in hypovolaemic shock. Do not use albumin or Haemaccel (polygeline). Dextrose solutions may cause hyperglycaemia if infused rapidly and hyponatraemia if they contain <0.9% saline.
In states of hyponatraemia or hypernatraemia, the correction of serum sodium should not occur at a rate >0.5 mmol/L per hour.
Replacement and maintenance therapy
Four basic aspects are considered:
- Existing deficit.
- Losses during therapy.
- Maintenance requirements.
- Principles of safe fluid management.
Existing defcit
Fluid defcit
The fluid deficit is most reliably estimated from the loss of body weight if a recent pre-illness weight is available. Otherwise the degree of dehydration can be estimated by the following:
Table 5.1 Fluid distribution
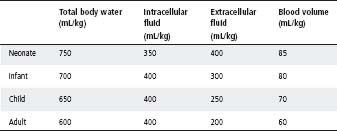
- Decreased perfusion (pallor or reduced central capillary return; hypotension is a late sign).
- Deep (acidotic) breathing – not useful in diabetic ketoacidosis.
- Decreased skin turgor.
- Increased thirst.
Weak markers of mild–moderate dehydration include a history of oliguria, restlessness, lethargy, sunken eyes, dry mouth, sunken fontanelle and the absence of tears.
Hence, if the bodyweight loss is
- <3–4%: no clinical signs
- 4–6%: clinical signs are present and the number and severity is a guide to the degree of dehydration
- ≥7%: hypotension and acidosis may be present.
In general, fluids to replace the existing deficit are given over the first 24 h. But, for hypernatraemic or hyponatraemic dehydration, they should be given over at least 48–72 h.
Electrolyte defcit
The type and degree of electrolyte deficit depends on the diagnosis. For example, in gastroenteritis, the loss of base leads to acidosis; in pyloric stenosis, the loss of acid and chloride leads to hypochloraemic alkalosis.
The rate of infusion of potassium should rarely be >0.2 mmol/kg per hour (and never >0.4 mmol/kg per hour). Concentrations of potassium >40 mmol/L should be used with extreme caution, generally only through a central line. Infusions of potassium at concentrations of ≥60 mmol/L should only be given in the intensive care unit. ECG monitoring should be considered in massive potassium replacement and infusions of concentrated solutions should be controlled with the use of a pump.
Continuing losses
Fluid balance charts should accurately record volumes of vomitus, gastric aspirates, drainage from fistulae, diarrhoea, urine output and other fluid losses. These parameters will determine both the volume and type of fluid and electrolyte replacement (see Table 5.5). Weights should be measured at least daily, as they are an important marker of ongoing losses.
Maintenance requirements
Maintenance fluid requirement is the daily water requirement for well children to excrete an iso-osmotic urine. The volumes required to achieve this in disease states are different from those in well children. See Tables 5.2 and 5.3.
Many acutely ill children have high levels of antidiuretic hormones (ADH), resulting in reduced free water excretion. This is termed Syndrome of ‘Inappropriate’ ADH secretion (SIADH), occurring particularly in postoperative children and those with brain or lung disease (meningitis, encephalitis, bronchiolitis, pneumonia). Hence, the total fluid required to maintain normal intravascular volume in these children is reduced (note that the aim is not to ‘dry out’ the patient). If these children are given standard maintenance fluid i.v., tissue oedema and hyponatraemia may occur. Hence, in these children give a total fluid intake (TFI) of about 60% of the usual maintenance volumes in Tables 5.2 and 5.3.
In contrast, some sick children will have increased fluid requirements (e.g. those with high fever, ongoing vomiting and diarrhoea, capillary leak or third-spacing of fluid into the abdomen). These children may need more than the standard maintenance fluid to maintain normal intravascular volume. Prescribing fluid volumes can be complex; some ill children have clinical features that imply a need for increased and decreased fluids. These children need regular assessments to ensure adequate hydration is achieved.
Principles of safe fluid management
Calculate the total fluid intake
At least once a day the TFI in mL/h and in mL/kg per day should be calculated. The prescribed TFI should include all fluid intake the child receives: i.v. deficit replacement + maintenance + estimation of continuing losses.
As the total fluid volume must not be exceeded, remember to factor in the following:
- i.v. fluid for drug lines
- blood products
- enteral feeds
- other.
Table 5.2 Daily maintenance intravenous fluid requirements
| Bodyweight | Requirements |
| 3–10 kg | 100 mL/kg per day |
| 10–20 kg | 1000 mL + (50 mL/kg per day for each kg over 10 kg) |
| 20 kg and over | 1500 mL + (20 mL/kg per day for each kg over 20 kg) |
Table 5.3 Hourly maintenance intravenous fluid requirements

Table 5.4 Adjustments to maintenance intravenous fluid requirements
| Condition | Adjustment to fluid intake |
| Renal failure | ×0.2 + urine output |
| Basal state | ×0.7 |
| High ADH (e.g. brain injury, meningitis) | ×0.7 |
| Breathing humidified gas | ×0.75 |
Table 5.5 Composition of some body fluids in children
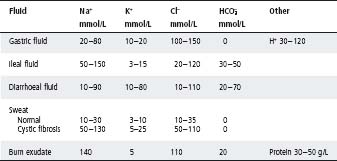
Hospitalised children on i.v. fluids often receive more than intended because other fluids (as listed above) have not been included in the calculation. The balance of enteral and parenteral fluid should be adjusted around this prescribed TFI. See Tables 5.2–5.5.
Regular monitoring
The key to good fluid management is regular clinical monitoring. Signs of dehydration should be corrected, but signs of overhydration, such as rapid weight gain and eyelid oedema, avoided.
- Children receiving i.v. fluids must be weighed every day. An increase in weight of 5% or more over 24 h indicates fluid overload. Manage by stopping i.v. fluids, measure serum sodium and consult senior medical staff. A decrease in weight of 5% or more indicates dehydration. Children receiving deficit replacement fluid should be weighed every 6 h.
- Assess for oedema every day. Check for eyelid and lower limb swelling. If either is present, stop i.v. fluids and consult senior medical staff.
- Children receiving i.v. fluids should have serum electrolytes checked at least daily. Senior medical staff should be consulted if:
– serum sodium is ≤130 mmmol/L or has fallen by >5 mmol/L
– serum sodium is ≥150 mmol/L or has risen by >5 mmol/L.
- For infants receiving i.v. fluids, consider measuring blood glucose every 6 h. If BSL is <3 mmol/L, increase glucose concentration of i.v. fluid from 5% to 10%.
The maintenance of pH within narrow limits is a result of two mechanisms:
- Buffer systems: the bicarbonate system is quantitatively the most important in plasma (70% of total).
- Excretory mechanisms: via the kidneys and lungs.
Acidosis (low pH) and alkalosis (high pH) may be respiratory or metabolic in origin. Respiratory disorders result from changes in the excretion of volatile carbon dioxide and, consequently, in the levels of carbonic acid. Metabolic disorders occur with changes in concentrations of non-volatile acids and, consequently, in the concentration of buffer base – mainly bicarbonate.
The four primary disorders are:
- Metabolic acidosis. This arises from increased production of non-volatile acid (e.g. ketoacids and lactic acid), failure of the kidney to excrete non-volatile acid or conserve base, or excess loss of buffer base (e.g. gastroenteritis or intestinal fistula).
- Metabolic alkalosis. This arises from excess loss of non-volatile acid (e.g. pyloric stenosis), excess intake of buffer (e.g. bicarbonate infusion), or potassium depletion where an extracellular alkalosis occurs as hydrogen ions are lost in the urine (e.g. renal tubular syndromes, pyloric stenosis or diuretic therapy).
- Respiratory acidosis. Hypercapnia is the result of alveolar hypoventilation from any cause (e.g. central, neuromuscular or pulmonary).
- Respiratory alkalosis. This is caused by hyperventilation.
Indicators of acid–base status
pH
This reflects the actual hydrogen ion concentration and alters in response to both respiratory and metabolic changes.
PCO2 (partial pressure of carbon dioxide)
In arterial and capillary blood samples, this represents the PCO2 in blood leaving the lungs. Alterations reflect disturbance in the respiratory component of the acid–base state. Changes may also reflect respiratory compensation for primary metabolic acidosis or alkalosis.
Base excess
This is an estimate of the change in total buffer base that would be present if the PCO2 was normal (40 mmHg). Change in the base excess reflects disturbance in the metabolic component of the acid–base status (not respiratory). In metabolic alkalosis the total buffer base is increased, and thus the base excess is positive. Other calculated measures of the metabolic component are the standard bicarbonate and the total buffer base.
Actual bicarbonate
This is the plasma concentration of bicarbonate; it is influenced by both metabolic and respiratory components.
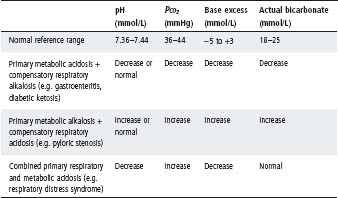
In practice, isolated metabolic and respiratory disorders are uncommon. Most disorders have a metabolic and a respiratory component. Usually one is the primary disorder and the other occurs secondarily and tends to correct the change in pH. However, sometimes primary metabolic and respiratory disturbances occur together; e.g. in respiratory distress syndrome, acidosis is contributed by both hypoxia leading to lactic acid accumulation and subsequent metabolic acidosis, whilst carbon dioxide retention results in respiratory acidosis (see Tables 5.6 and 5.7).
The interpretation of changes in base excess and PCO2 as primary or secondary changes must be made clinically.
Correction of metabolic acidosis
Metabolic acidosis often resolves rapidly once the cause has been corrected and administration of bicarbonate is usually not required. Consider giving bicarbonate if there is marked metabolic acidosis (large base deficit, normal PCO2) with a pH of <7.10. Give bicarbonate for metabolic acidosis with a pH <7.15 caused by bicarbonate loss, which is characterised by a normal anion gap of <12 mmol/L (anion gap = Na − bicarbonate − Cl).
- The amount of bicarbonate required is guided by:
– mmol of HCO3 required = base deficit (mmol/L) × weight (kg) × 0.3
- It is usual to give only half the amount of bicarbonate suggested by this formula, then repeat the blood gas measurement before giving more.
- The factor (weight × 0.3) represents the volume of extracellular fluid through which the base deficit (negative base excess) is distributed.
Table 5.6 Changes in arterial capillary blood (before compensation)
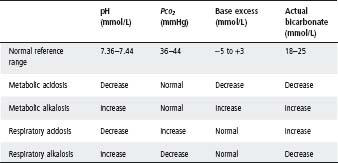
Table 5.7 Change in indicators of acid–base status seen in combined disorders
- In babies <5 kg, the factor is weight × 0.5 because of the greater percentage of extra-cellular fluid.
The rate of administration of bicarbonate will be determined by the cause of the metabolic acidosis and the clinical state of the patient; e.g. in cardiac arrest infuse bicarbonate rapidly; if acidosis has been present for >6 h, the correction should usually be undertaken over at least 6 h (and mild to moderate acidosis will resolve with rehydration alone). Rapid correction of metabolic acidosis will cause a fall in extracellular potassium.
The principles of correction of metabolic alkalosis are discussed in the following section on pyloric stenosis.
Management of some special conditions
Pyloric stenosis
All infants with pyloric stenosis should have their acid–base and electrolytes measured. Persistent vomiting results in predominantly hydrogen and chloride loss, with consequent hypochloraemic alkalosis. Losses of potassium in vomitus and urine can lead to significant hypokalaemia. In severe hypokalaemia, potassium is conserved in the kidneys preferentially to hydrogen ions, leading to paradoxical aciduria.
The duration and severity of vomiting will, to a large extent, determine the degree of fluid and electrolyte imbalance.
Assessment
All patients require biochemical assessment, but not all require i.v. therapy preoperatively. Any child who is clinically dehydrated or has significant biochemical derangement requires i.v. fluids.
I.v. therapy
- In severe dehydration (≥7%), commence with 0.9% saline until adequate circulation has been established and continue with 0.45% saline and 5% dextrose (see Table 5.11).
- In moderate dehydration (4–6%), commence with 0.45% saline and 5% dextrose (see Table 5.11).
- In mild dehydration, await electrolyte results before commencing therapy.
- Adequate potassium and chloride replacement is necessary to correct the acid–base abnormality. As soon as urine flow is established, potassium chloride is given up to a maximum of 0.4 mmol/kg per hour. Usually 20–40 mmol/L is adequate.
Acute oliguria and anuria
Severe oliguria is a urine output of <0.5 mL/kg per hour.
Initial management
Three clinical situations occur:
- Sudden and complete anuria should arouse suspicion of urinary obstruction. Ensure the catheter is correctly placed and not blocked.
- Renal hypoperfusion causing oliguria should be suspected in the presence of clinical dehydration or septicaemia leading to hypotension; the urinary sodium is usually <20 mmol/L. Blood volume expansion is necessary.
- Renal tissue injury (e.g. acute poststreptococcal glomerulonephritis or secondary to hypoperfusion or nephrotoxin). Urinary sodium is usually >40 mmol/L. Give i.v. frusemide (1–2 mg/kg) and, if there is no response, institute treatment for continuing anuria.
Continuing anuria
Fluid intake is restricted to the previous 24 h urine output and any abnormal losses; approximately 20% of normal maintenance requirements must also be given to replace insensible water loss.
At least 20% of full energy requirements is given as carbohydrate to spare tissue protein from catabolism for energy, which accentuates the rise in blood urea. I.v. 10–20% glucose, oral Caloreen or Polyjoule are suitable.
Peritoneal dialysis or haemofiltration will be required for hyperkalaemia, severe uraemia (blood urea ≥50 mmol/L), acidosis, or water overload leading to hyponatraemia, oedema or hypertension. It should also be considered if anuria persists for >24 h.
Hyperkalaemia
The management of hyperkalaemia depends on the underlying cause. Serum potassium is increased by acidosis and reduced by alkalosis. In acute oliguric renal failure, if urine is not formed after i.v. frusemide (see above), the presence of hyperkalaemia (≥7.0 mmol/L) is an indication for dialysis. High values (up to 7.0 mmol/L) not requiring treatment may be found in the neonatal period. Obtain an urgent 12-lead ECG – peak T waves suggest that treatment is needed, especially if the QRS complex is widened.
If cardiac arrhythmias are present, rapid but temporary benefit may be achieved by giving (while arranging dialysis):
- I.v. sodium bicarbonate 2 mmol/kg.
- Short-acting (regular) insulin 0.1 unit/kg given with 2 mL/kg of i.v. 50% dextrose.
- I.v. calcium gluconate 10% 0.5 mL/kg, given slowly.
- Rectal or NG sodium polystyrene sulfonate (Resonium) 1 g/kg.
Hypokalaemia
Metabolic alkalosis may cause hypokalaemia, but this resolves if alkalosis is treated. Severe hypokalaemia causes long QT interval, often with prominent U waves. Management is usually sufficient with supplementation of oral potassium or by adding it to i.v. maintenance fluid. Potassium should rarely be given faster than 0.2 mmol/kg per hour and never faster than 0.4 mmol/kg per hour even in ICU. Concentrated potassium infusions can cause chemical burns if extravasation occurs, hence the need for reliable central line access.
Hypernatraemia
If the patient is ‘shocked’, provide volume resuscitation with 0.9% saline as required in 20 mL/kg boluses. The aim is to lower the serum [Na+] slowly at a rate of no faster than 12 mmol/L in 24 h (0.5 mmol/L per hour). If sodium is corrected too rapidly in hypernatremia, cerebral oedema, seizures and permanent brain injury may occur. Monitor and manage for concurrent hyperglycaemia and hypocalcaemia.
Moderate hypernatraemic dehydration, [Na+] 150–169 mmol/L
After initial resuscitation, replace the deficit plus maintenance over 48 h.
- Use nasogastric oral rehydration solution (Gastrolyte), but remember that Gastrolyte has a sodium concentration of 60 mmol/L.
- If needing i.v. rehydration use 0.45% NaCl + 5% dextrose or 0.9% NaCl + 5% dextrose. Add maintenance KCl once urine output established.
Daily volume (mL) = Daily maintenance fluids (mL) + remaining fluid deficit (mL)/2 Rate (mL/h) = Daily volume (mL)/24(h)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


