Drug
Formulation
Delivery system
Dosage form
Release location
Azo-bonded formulations
Sulfasalazine (Azulfidine®)
Azo bond of 5-ASA to sulfapyridine
Broken down by colonic bacteria to release active 5-ASA moiety
Tablet 500 mg
Colon
Osalazine (Dipentum®)
Azo bond of 5-ASA to a dimer
Broken down by colonic bacteria to release active 5-ASA moiety
Capsule 250 mg
Colon
Balsalazide (Colazal®)
Azo bond of 5-ASA and a carrier
Broken down by colonic bacteria to release active 5-ASA moiety
Capsule 750 mg
Colon
Mesalamine formulations
Pentasa®
Controlled release
Time release
Capsules 250 mg, 500 mg
Small intestine, colon
Asacol®
Enteric coated; delayed release
pH-dependent (≥7)
Tablet 400 mg
Terminal ileum, colon
Asacol HD®
Enteric coated; delayed release
pH-dependent (≥7)
Tablet 800 mg
Terminal ileum, colon
Lialda®
Delayed release
pH-dependent (≥7)
Tablet 1,200 mg
Terminal ileum, colon
Apriso™
Delayed and extended release
pH-dependent (≥6)
Capsule 375 mg
Terminal ileum, colon
Rowasa®
Topical
Rectal suspension 4 g/60 mL
Left colon
Canasa®
Topical
Suppository 1,000 mg
Rectum
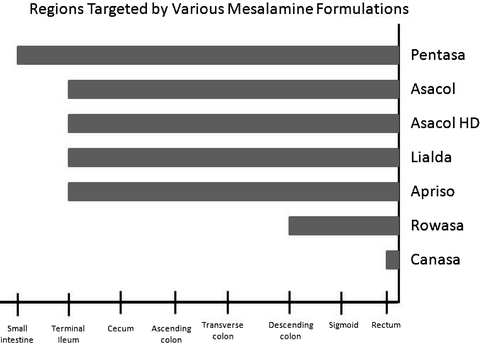
Fig. 26.1
Regions targeted by various mesalamine formulations
Indications and Efficacy
Ulcerative Colitis
The efficacy of aminosalicylates for the induction and maintenance of remission of ulcerative colitis (UC) is well established in the adult literature and these medications are used as first-line treatment for mild-to-moderate disease [18, 19]. Conversely, there is very little pediatric UC data and there are no randomized controlled trials on aminosalicylates maintaining remission of UC in pediatric patients. In a recent systematic review and meta-analysis, both oral and rectal preparations of 5-ASAs were found to have modest efficacy at inducing remission in mild-to-moderate UC compared to placebo with no statistically significant difference between the preparations [20]. There is no standardized dosage or frequency of dosing for rectal preparations in inducing remission of UC. In the most recent Cochran Review, rectal 5-ASA was superior to rectal steroids for inducing remission of UC [21]. There is improved efficacy with combined 5-ASA therapy including rectal and oral preparations compared with oral 5-ASA therapy alone [22]. A significant reduction in the risk of failure to induce remission with a number needed to treat (NNT) of 5 for the combined 5-ASA therapy was observed. There is no standard dosing of oral 5-ASA for inducing remission. Doses of 1.5–4.8 g/day have been shown to be effective depending on disease severity. The results of the ASCEND I and II trials show a statistically significant higher rate of mucosal healing in UC at 6 weeks with a dose of 4.8 g/day of delayed-release oral mesalazine over 2.4 g/day dosing [23]. Despite improved efficacy of combined 5-ASA therapy for inducing remission over oral 5-ASA alone, the remission rates are still significantly lower than with corticosteroids alone [21]. In UC, mesalamine has similar efficacy to sulfasalazine at equimolar doses.
Both oral and rectal mesalamine are more efficacious in preventing relapse of quiescent ulcerative colitis than placebo [24]. There are many randomized control trials that show topical 5-ASAs have comparable efficacy at preventing relapse of quiescent UC. On the other hand, in one recent meta-analysis, intermittent rectal mesalamine was superior to oral 5-ASAs with an NNT of 4 [22, 25]. In another recent meta-analysis, topical mesalamine was more effective at preventing relapse of quiescent UC compared to placebo with an NNT of 3 [26]. This study also showed a trend toward a greater effect size with continuous topical therapy compared with intermittent topical therapy. The analysis showed lower relapse rates when an overall higher total weekly dose of topical mesalamine was used, similar to the occurrence with higher doses of oral 5-ASA therapy for preventing relapse of quiescent UC. However, the majority of the patients in this study had only left-sided disease or proctitis.
Many studies have shown the efficacy of oral 5-aminosalicylic acid in maintaining remission of quiescent ulcerative colitis [24]. In the adult population, oral 5-ASA has modest efficacy with good adherence but there is no standardized dosing regimen. Some of the more recent studies are not only looking at the efficacy in maintaining remission in UC, but also at the adherence to the prescribed treatment. In one recent study of MMX mesalamine at 2.4 g/day, there was only a 30% recurrence rate at 12 months for patients that were adherent to the medication more than 80% of the time, as compared to a 53% relapse rate at 12 months for patients who were less than 80% adherent to the medication regimen [27]. A meta-analysis showed that once-daily dosing of oral mesalamine was equally as effective as conventional dosing in preventing relapse in quiescent UC over 12 months of therapy [28, 29]. Although 5-ASA has proven to be effective in maintaining remission in quiescent ulcerative colitis, adherence must be considered when developing an individual’s treatment plan.
As is common with many IBD therapies, there are no randomized control trials for 5-ASA in the pediatric population. In fact, there are few studies evaluating the efficacy of 5-ASA for the treatment and maintenance of remission in pediatric UC. There is one recent study on the efficacy of mesalamine suppositories for the treatment of ulcerative proctitis in children. This study measured disease activity index of pediatric patients with ulcerative proctitis while receiving mesalamine suppositories 500 mg at bedtime. In the 49 patients enrolled, the data showed a statistically significant decrease in the disease activity index for patients at 3 weeks. Forty-one patients had a mild or unrelated adverse event [30]. Another recent pediatric study compared the efficacy of oral beclomethasone dipropionate (BDP) to oral 5-ASA in the treatment of mild-to-moderate UC in the pediatric population. The results of the study showed clinical remission was achieved after 4 weeks in 12 of 15 patients treated with BDP but only 5 of 15 patients treated with 5-ASA, thus showing BDP more efficacious at inducing remission in mild-to-moderate pediatric UC than 5-ASA [31]. Dosing of oral 5-ASA in the pediatric population is variable, but the dosages usually fall in the range of 30–100 mg/kg/day with a maximum dose of 4 g/day.
Crohn Disease
Efficacy of 5-ASA inducing and maintaining remission in Crohn disease is controversial. There are no randomized control studies examining the induction of remission or maintenance of remission by aminosalycilates in pediatric Crohn disease. In a recent Cochrane review consisting of adult studies, sulfasalazine showed only a modest effect over placebo in inducing remission in mild-to-moderate Crohn disease at a dose of 3–6 g/day [32]. It showed a 38% higher chance of inducing remission compared to placebo-treated patients. However, this effect is limited to patients with Crohn’s colitis. Sulfasalazine was 34% less effective at inducing remission than corticosteroids alone and it was less effective than combination therapy with corticosteroids and sulfasalazine. Two studies, the Trial of Adjunctive Sulfasalazine in Crohn disease (TAS) and the European Cooperative Crohn Disease Study (ECCDS), showed that sulfasalazine was not a useful adjunct to corticosteroid therapy in achieving remission [33, 34].
A recent systematic review and meta-analysis of randomized controlled trials that excluded the Crohn’s III trial data, also suggests a modest effect of 5-ASA drugs inducing remission in Crohn disease over placebo-treated patients with an NNT to prevent one patient’s disease remaining active of 11 [35]. The effect was based on a mean reduction in CDAI scores. Had the data from the Crohn’s III trial been available, the review suspects there would have been no statistically significant difference between the 5-ASA treated group and the placebo-treated group.
According to the latest Cochrane review, low-dose controlled-release mesalamine (1–2 g/day) was less effective at inducing remission in active Crohn disease compared to placebo-treated patients [32]. As with sulfasalazine, delayed-release mesalamine (2 g/day) was less efficacious than corticosteroids [36]. Trials evaluating higher doses of measalamine (3–4.5 g/day) show inconsistent results. The majority of the studies show no difference in induction of remission in mild-to-moderately active Crohn disease relative to the placebo [32]. Two of the studies showed statistically significant changes in CDAI scores but they were found to be clinically insignificant. In a single trial, high-dose mesalamine was less effective than budesonide [37]. Many of these studies were small and had several methodological weaknesses, which may limit the generalizability of the effects of mesalamine at inducing remission in mild-to-moderately active Crohn disease.
One pediatric study reviewed disease activity at diagnosis in 43 patients and treatment provided. Ten of 25 patients in the mild group and 3 of 18 patients in the moderate-to-severe group received 5-ASA monotherapy immediately after diagnosis. However, these patients tended to have more exacerbations, shorter duration of the first remission, and longer total duration of systemic steroid use than patients receiving combination therapy, immunomodulators, or systemic steroids [38].
The role of 5-ASAs at maintaining remission in quiescent Crohn disease was also assessed and there appears to be no statistically significant difference between 5-ASAs and placebo [35, 39]. When only trials with low risk bias were analyzed, 5-ASAs appeared to be more effective at preventing relapse than placebo with an NNT of 13 [35]. This was the same result when a more conservative protocol analysis was completed, in which dropouts from individual studies were not considered treatment failures. The effectiveness of salfasalazine and mesalamine at maintaining remission in quiescent Crohn disease is similar and still unclear [39].
The role of aminosalicylates for inducing and maintaining remission in mild-to-moderately active Crohn disease is controversial and all the studies have heterogeneity in clinical design, dosing, and preparation. Many gastroenterologists continue to use aminosalicylates in Crohn disease despite many studies showing at best a modest benefit over placebo [32]. As for the use of aminosalicylates in the maintenance of remission, there is currently no good data to support its use [39].
Surgically Induced Remission of Crohn Disease and Prevention of Postoperative Recurrence
Surgical resection can induce remission in Crohn disease. However, endoscopic and clinical relapse of Crohn disease after surgical resection is common and can be as high as 75% and 20%, respectively, within 1 year [40]. There is currently no standard therapy for preventing relapse post-operatively. Aminosalycilates in the postoperative setting have been extensively studied, but their effectiveness at preventing relapse after surgical resection remains controversial. In a recent systematic review and meta-analysis of 11 randomized controlled trials, the effect of mesalamine appears to be modest with an NNT of 13 compared to placebo or not treating after surgery [41]. In a Cochrane review, the effectiveness of mesalamine was even more modest with an NNT of 16–19 [42]. However, this effect seems to be limited to mesalamine only, as sulfasalazine demonstrated no advantage over the control therapy. There is heterogeneity in all these studies including the dosage used, preparation used, length of treatment post surgery, and definition of remission. Based on these results, one could consider mesalamine for prevention of relapse after surgical resection when immunosuppressive therapy is contraindicated [41, 42].
Chemoprevention of Colorectal Carcinoma
For many years, people have believed that the use of 5-ASAs greatly reduced the risk of colorectal carcinoma (CRC) in patients with IBD based on their structural similarity to aspirin, which has been shown to reduce the risk of developing CRC and adenomas in patients without IBD [43]. However, more recent studies are finding that it might not provide much, if any, chemoprophylaxis of CRC. A recent population-based study including more than 8,000 patients found no protective effect of 5-ASA against CRC [44, 45]. This study looked at the cumulative use of 5-ASA at 1, 5, and 7.5 years and looked at a case–control study of patients with CRC compared to a matched group without CRC. Adherence to 5-ASA therapy was based on the frequency of prescription refills. However, it is possible that the cumulative use of more than 7.5 years could be chemoprotective, but no study has been done looking this far out. Also, a large study from the UK showed non-adherence to 5-ASA in patients with CRC represented non-adherence to other healthy behaviors that might have put them at increased risk for cancer [46]. In contrast, one small case-controlled study found that cumulative mesalamine doses decreased the risk of CRC in patients with IBD [47]. There are also several studies that have observed a significant chemopreventive effect of mesalamine compounds, especially at doses of >1.2 g/day. However, these studies have been criticized because of the design, outcomes measured, and variables controlled for [48]. Most studies agree that mesalamine has a more protective effect compared with sulfasalazine, but a large multicenter prospective randomized control trial to fully study 5-ASA as a chemoprophylatic has not been completed [49].
The exact mechanism of action of 5-ASA to treat IBD is unknown, and the same can be said regarding chemoprophylaxis. There is one retrospective cohort study that has attempted to look at where in the dysplasia-carcinoma sequence they might work. The study identified patients with UC with no dysplasia, indefinite dysplasia, or flat low-grade dysplasia (LGD) and followed them for the development of high-grade dysplasia (HGD) or CRC. The data suggest that if mesalamine has any chemopreventive effect, it may act early in the neoplastic process before the development of LGD [48]. There are many in vivo and in vitro studies currently looking at the anti-inflammatory and antineoplastic effects on different proposed mechanism of action pathways, including inhibition of COX activity, enhanced apoptosis through inhibition of NF-κB and MAP kinases, improvement in the DNA replication process, inhibition of reactive oxygen species, and downregulation of oncogenes and transcription factors [43, 50]. Now that 5-ASA is thought to be involved in inhibition of protein synthesis, this may add to its anti-inflammatory and anti-neoplastic properties.
Side Effects
Sulfasalazine (SASP) therapy is usually accompanied with more side effects than the 5-ASA formulations due to the sulfapyridine moiety [3]. Up to 80–90% of patients who cannot tolerate sulfasalazine, tolerate 5-ASA preparations [51]. In addition, patients who experience adverse reactions to a particular 5-ASA formulation often tolerate a different preparation.
Side effects of 5-ASA are listed in Table 26.2. The most common side effects of both SASP and 5-ASA, are nausea, abdominal pain, diarrhea, dyspepsia, rash, and fever [52, 53]. Some of these effects, such as diarrhea, can be mitigated by a gradual increase in the dose [54]. Rare, but more serious side effects include interstitial nephritis, pancreatitis, pericarditis, pneumonitis, hepatitis, neutropenia, and rarely worsening colitis [51–53]. The risk of interstitial nephritis and pancreatitis is higher with 5-ASA, while the risk of hepatitis is higher with SASP. Agranulocytosis, hemolytic anemia, and oligospermia have been reported with SASP [53].
Table 26.2
Side effects of 5-ASA and sulfasalazine
5-ASA | Sulfasalazine |
|---|---|
Common
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |