Rational antimicrobial prescribing
- Unnecessary antibiotic use for viral illnesses contributes to the increasing problem of antibiotic resistance. Most respiratory tract infections in children, including tonsillitis and otitis media, are self-limiting and do not require antibiotic therapy. If the diagnosis is unclear, it is preferable to repeat the clinical evaluation and simple laboratory tests, rather than use empiric antibiotic therapy ‘just in case’.
- Antibiotics do not prevent secondary bacterial infection in viral illnesses.
- The use of antibiotics may make definitive diagnosis and subsequent decisions about management more difficult.
- Empiric antibiotic therapy (i.e. not based on specific aetiological diagnosis) should only be prescribed when a serious bacterial infection is suspected (e.g. meningitis) and it is not safe or possible to obtain definitive culture specimens or culture results are pending.
- Empiric therapy should be based on the likely cause, local antibiotic resistance patterns and individual host factors (e.g. immunocompromise) in accordance with local guidelines.
- For mild infections, the safest and best-tolerated antibiotic with the narrowest spectrum against the most likely pathogens should be chosen (e.g. trimethoprim for urinary tract infection).
- For serious infections, broad-spectrum agents are chosen until the pathogen and its susceptibility is identified (e.g. cefotaxime for meningitis).
- Theoretical benefits of new antibiotics based on in vitro data do not necessarily translate into greater efficacy. Newer antibiotics often offer no advantages, might be expensive with more side effects and have a greater likelihood of leading to resistance or superinfection.
Although many bacteria are still susceptible to long-established treatments, antibiotic resistance is an increasing problem worldwide. Examples of particular clinical concern include:
- Penicillin (and cephalosporin)-resistant Streptococcus pneumoniae (PRP).
- Methicillin (multidrug)-resistant Staphylococcus aureus (MRSA).
- Community-acquired non-multiresistant MRSA (CA-MRSA).
- Glycopeptide (vancomycin and teicoplanin) intermediate-resistant Staphylococcus aureus (GISA).
- Vancomycin-resistant Enterococcus (VRE).
- Multidrug-resistant Mycobacterium tuberculosis (MDRTB) and extensively drug-resistant tuberculosis (XDRTB).
- Bacteria that produce inducible b-lactamases (IBL) which are always present in bacteria of the ‘ESCHAPPM’ group comprising Enterobacter spp., Serratia marscesens, Citrobacter freundii, Hafnia spp., Aeromonas spp., Providencia spp., Proteus vulgaris and Morganella morganii.
- Bacteria that produce extended-spectrum â-lactamases (ESBL), e.g. some E. coli and Klebsiella spp. which are associated with cephalosporin (and often gentamicin resistance).
- Multidrug-resistant Salmonella spp.
- Macrolide-resistant Streptococcus pyogenes.
Infections with resistant organisms should be considered in patients who have had prolonged hospitalisation, known exposure to resistant organisms or failed response to initial empiric therapy. Strategies to deal with infections caused by these organisms include using new or broader-spectrum antibiotics, or alternatively using two or more antibiotics concurrently. The choice of empiric therapy becomes increasingly difficult. Specialist consultation is strongly advised.
Fever is the most common presenting symptom in children in the primary care setting. Although there is no universally accepted definition, fever is generally considered to be present if core temperature (rectal or tympanic) is >38 °C. Axillary and oral temperatures may underestimate body temperature by at least 0.5 °C.
Although tympanic thermometers provide certain advantages over other thermometers (ease of use, rapid results and convenience), several studies have found that they are not as accurate nor sensitive for the detection of fever, particularly in infants <3 months of age. Rectal (neonates), oral and axillary (neutropenic patients) temperatures are preferable for accurate fever detection.
Self-limiting viral infections are the most common cause of fever in children. However, the challenge to the clinician is to identify those children with a more serious cause. Fever in children may be classified into three groups:
- Fever with localising signs.
- Fever without focus.
- Fever (or pyrexia) of unknown origin.
Fever with localising signs
A careful history and examination will identify the source of infection in most patients. These children should be managed according to the individual condition and its severity.
Fever without focus
In a small number of children presenting with fever, no focus is found. Most will have a viral infection, but a more serious illness such as a urinary tract infection (5–8%), occult bacteraemia (<1%) or meningitis may be present. Infants (<12 months old) with rectal temperature >38.0 °C have a higher risk of occult bacteraemia (up to 15%).
Most children who present with fever and no identifiable focus appear otherwise well. History should include details about immunisation status, infectious contacts, travel, diet and contact with animals. A thorough physical examination should be carried out, paying particular attention to:
- General appearance: the level of activity and social interaction; peripheral perfusion and colour.
- Vital signs: pulse; respiration; blood pressure.
- Possible clues to source: full fontanelle, neck stiffness, photophobia; respiratory distress (tachypnoea; grunt; nasal flare; retractions), abnormal chest signs; rhinitis, pharyngitis, otitis or mastoiditis; lymphadenopathy; abdominal distension, tenderness or masses; hepatosplenomegaly; bone and joint tenderness or swelling; skin rashes, petechiae or purpura, or skin infection.
- Always consider Kawasaki disease in any child with a persistent fever. It is the only rare cause of persistent fever that requires urgent treatment.
Patients with unexplained fever with a higher likelihood for serious infection include the following patient groups or conditions:
- Neonates and infants <3 months of age.
- Immunocompromised (e.g. congenital immunodeficiency, HIV, neutropenic and other oncology patients, cytotoxic drugs and steroids).
- Asplenic children (congenital, post splenectomy or functional, e.g. sickle cell disease).
- Patients who have received prior oral antibiotics. Many of these patients have a viral infection, but meningitis or other serious bacterial infection must be considered.
- Children with central venous or arterial catheters, or other foreign bodies, including shunts.
- Multiple congenital abnormalities.
- Other specific illnesses (e.g. sickle cell disease, cystic fibrosis or structural cardiac defects (endocarditis)).
- Toxic-appearing children (e.g. those with an altered conscious state, decreased peripheral perfusion (check capillary refill centrally) or purpuric rash).
- Children <6 months of age (higher chance of UTI).
- Children <12 months of age with febrile convulsion, or any children with febrile convulsion lasting longer than 10 min (consider lumbar puncture to exclude meningitis).
These children require admission to hospital, with culture of blood, urine and CSF (‘full septic screen’) and a chest radiograph if indicated. Antimicrobial therapy should be based on the patient’s clinical illness and the local epidemiology of potential pathogens and their antibiotic susceptibility (see Antimicrobial guidelines).
Table 30.1 Management of fever without focus
| Age | Investigations | Management |
| <1 month | FBE; blood, urine and CSF cultures; CXR | • Admit |
| • Empiric i.v. benzylpenicillin and gentamicin, plus cefotaxime if meningitis is suspected (see Antimicrobial guidelines) | ||
| 1 to 3 months | As above (CXR may be omitted if no respiratory symptoms or signs present) | • If WCC 5–15 × 109/L with other investigations normal: discharge and review within 12 h, or sooner if deterioration occurs |
| • If child is unwell, or any results are abnormal: admit and consider empiric antibiotics (see Antimicrobial guidelines) | ||
| 3 months to 3 years and well | Consider urine culture (mandatory if <6 months) | • If <6 months and UTI is suspected from dipstick urine testing: admit for i.v. benzylpenicillin and gentamicin (see Antimicrobial guidelines) |
| • Otherwise: discharge and review within 24 h, or sooner if deterioration occurs | ||
| 3 months to 3 years and unwell | FBE; blood, urine and CSF cultures; CXR if respiratory symptoms or signs present | • Admit and start empiric antibiotics: |
| – if CSF normal: i.v. flucloxacillin and gentamicin | ||
| – if CSF abnormal or unavailable: i.v. cefotaxime (see Antimicrobial guidelines) |
Notes:
- Fever = rectal temperature >38 °C (>38.9 °C over 3 months of age).
- FBE = full blood examination, including film; CSF = cerebrospinal fluid; WCC = white cell count; CXR = chest X-ray.
- Urine specimens should be obtained by suprapubic aspiration or catheter drainage. Bag specimens are useless in this context.
- Lumbar puncture should not be performed in a child with impaired conscious state or focal neurological signs (see chapter 1, Medical emergencies, chapter 3 Procedures).
- Ceftriaxone can be substituted for cefotaxime (see Antimicrobial guidelines).
In the absence of the above risk factors, a febrile child >6 months of age who appears well but without a focus of infection does not require laboratory testing or treatment, though a urine microscopy and culture may be appropriate.
There is no evidence that oral or parenteral antibiotics prevent the rare occurrence of focal infections from occult bacteraemia; instead, they result in delayed diagnosis, drug side effects, additional costs and the development of resistant organisms. What is required is a careful clinical assessment, review within 24 h and parental education. See Table 30.1 and Box 30.1 on p. 384.
Occult bacteraemia
Infants with bacteraemia may clear the bacteria spontaneously. This is particularly true for pneumococcal bacteraemia. Patients who grow Streptococcus pneumoniae in their original blood culture do not require further investigation or treatment if they are now well, remain afebrile and have not received antibiotics, as they have cleared the organism independently. Parents should, however, be asked to bring children back for immediate review if they develop further fever within the following 7 days. Refer for specialist advice if uncertain.
Other pathogens causing occult bacteraemia should be treated with appropriate antibiotics.
Box 30.1 Advice for parents about fever
When caring for your child:
- Make the child comfortable, e.g. dress in light clothing.
- Give small, frequent drinks of clear fluid, e.g. water or diluted juice.
- Fever does not necessarily require treatment with medication. Finding the cause and treating it is often more important.
- Paracetamol may be given if the child is irritable, miserable or appears to be in pain (15 mg/kg p.o. 4 hourly when required, to a maximum of 90 mg/kg per day).
- Giving paracetamol has not been shown to prevent febrile convulsions.
- Do not continue giving regular paracetamol for more than 48 hours without having the child assessed by a doctor.
- Avoid aspirin and other NSAIDs:
– Aspirin because of risk of Reye’s syndrome.
– NSAIDs because of potential association with invasive staphylococcal and streptococcal disease (including necrotising fasciitis).
Seek immediate medical attention if there is no improvement in 48 hours or at any time if your child:
- Looks ‘sick’: pale, lethargic and weak.
- Suffers severe headache, neck stiffness or complains of light hurting their eyes.
- Has breathing difficulties.
- Refuses to drink anything.
- Persistently vomits.
- Shows signs of drowsiness.
- Suffers pain.
Partially treated bacterial infection
Patients presenting with fever who have received prior antibiotics should be assessed with a high index of suspicion. Although the child may have a viral illness, partial treatment with antibiotics may mask the typical clinical presentation of a serious bacterial infection, such as meningitis. A full septic screen should be considered even if the child looks well. For this reason, neonates should almost never be treated with oral antibiotics in the community.
Pyrexia (fever) of unknown origin
Pyrexia of unknown origin (PUO) is defined as prolonged fever (usually accepted as 2 weeks or longer) for which history, examination and initial investigations have failed to reveal a cause. In general, PUO in children is more likely to be due to chronic, non-infectious conditions, such as juvenile chronic arthritis and other collagen vascular diseases, inflamatory bowel disease or malignancy. Infectious causes include systemic viral syndromes (such as infectious mononucleosis), upper or lower respiratory infections (e.g. sinusitis), urinary tract infection, CNS infection, bone infection, tuberculosis, abscess (e.g. parameningeal, intraabdominal), endocarditis and enteric infections (e.g. typhoid fever). The term PUO is often incorrectly applied to patients who are suffering a series of simple viral infections.
Febrile neutropenia
See chapter 29, Haematologic conditions and oncology.
Group A streptococcus
Group A â-haemolytic streptococci (GABHS or Streptococcus pyogenes) cause a variety of diseases including pharyngotonsillitis (see chapter 24, Ear, nose and throat conditions), impetigo, cellulitis, scarlet fever, otitis media, streptococcal toxic shock syndrome, necrotising fasciitis, glomerulonephritis and rheumatic fever. Group A streptococcal pharyngitis is extremely uncommon in children <5 years of age. S. pyogenes is currently always sensitive to penicillin.
Scarlet fever
- Transmission: droplet, direct contact.
- Incubation period: 2–5 days.
- Infectious period: 10–21 days (24–48 h, if adequate treatment).
Clinical features
- Prodrome: sudden-onset high fever, vomiting, malaise, headache and abdominal pain.
- Rash: appears within 2 h of prodrome, diffuse red flush involving torso and skin folds, blanches, circumoral pallor, strawberry tongue (initially white, then red day 4–5), pharyngotonsillitis, tender cervical/submaxillary nodes.
Complications: otitis media, retropharyngeal abscess, quinsy, rheumatic fever, glomerulonephritis.
Diagnosis: culture of throat swab may confirm clinical impression
Treatment:
- phenoxymethylpenicillin (penicillin V) 250 mg p.o. (<10 years), 500 mg p.o. (>10 years) 12 hourly for 10 days.
- Control of case: exclude from school until treated for 24 h.
- Differential diagnosis: Kawasaki disease, streptococcal or staphylococcal toxic shock syndrome, viral infection
Acute rheumatic fever
- Incubation period: 7–28 days after group A streptococcal infection.
Clinical features
Jones criteria (1992) for initial diagnosis: two major, or one major plus two minor manifestations, plus evidence of antecedent group A streptococcal infection by culture or serology.
- Major manifestations:
– Carditis (usually mitral regurgitation murmur).
– Polyarthritis.
– Erythema marginatum.
– Subcutaneous nodules.
– Chorea (does not require evidence of recent GAS infection).
- Minor manifestations:
– Fever.
– Polyarthralgia or aseptic mono-arthritis.
– Raised inflammatory markers (ESR ≥30 mm/h or CRP ≥30 mg/L).
– Prolonged PR interval on ECG.
Recurrences may be diagnosed with three minor manifestations plus evidence of antecedent group A streptococcal infection without major manifestations, if there are no other more likely diagnoses.
Complications
Increased risk of recurrent disease, particularly for first 5 years after an attack. Heart valve damage may be permanent, especially after severe or recurrent disease, leading to rheumatic heart disease.
Diagnosis
Clinical features (Jones criteria) + culture/serology. Echocardiography may be useful in detecting subclinical lesions or typical rheumatic valvular involvement.
Treatment
- Admission to hospital.
- Phenoxymethylpenicillin (penicillin V): 250 mg p.o. (<10 years), 500 mg p.o. (>10 years) 12 hourly for 10 days or a single i.m. injection of benzathine penicillin G (900 mg, or 450 mg if <30 kg).
- Aspirin:
– Weeks 1 and 2: 25 mg/kg p.o. 6 hourly (4–8 g/day total in adults) initially. – Weeks 3 and 4: Reduce to 15 mg/kg p.o. 6 hourly.
– May be used to relieve arthritis or fever and leads to resolution of symptoms within 1–2 days (sometimes this response is diagnostic of rheumatic fever).
– There is no evidence that aspirin affects the long-term outcome.
- Corticosteroids (usually prednisolone 2 mg/kg per day, tapering after 2 weeks) are often used when carditis with cardiac failure is present, although there is no definitive evidence that they improve long-term outcome.
- For severe chorea, haloperidol, sodium valproate or other major tranquillisers have been used with mixed success.
Follow-up
Secondary prophylaxis is essential to prevent subsequent group A streptococcal infections, which may cause recurrences. Phenoxymethylpenicillin (penicillin V) 250 mg p.o. twice daily, or benzathine penicillin G 900 mg i.m. every 3 or 4 weeks. Duration is until 21 years of age or 5 years after last attack (whichever is later) or to age 35 years if moderate or severe carditis present. Prophylaxis may be lifelong if severe carditis or if patient has required cardiac surgery. Long-term clinical and echocardiographic follow-up is essential.
Post-infectious glomerulonephritis
See chapter 35, Renal conditions.
Streptococcus pneumoniae
Streptococcus pneumoniae (pneumococcus) is a Gram-positive coccus (usually appearing as diplococci) that causes a wide variety of infections including severe, invasive disease (e.g. meningitis, septicaemia, septic arthritis, peritonitis), or mild, often self-limited, invasive disease (occult bacteraemia), pneumonia, otitis media and sinusitis.
Transmission
Droplet.
Epidemiology
The epidemiology of pneumococcal disease in children is changing as a result of the routine use of conjugate pneumococcal vaccine. In trials, the vaccine provided ∼70% protection against invasive disease (meningitis, septicaemia), ∼50% protection against radiographically proven pneumonia, and <5% protection against otitis media caused by Pneumococcus. Routine use of this vaccine in the USA has led to a large decrease in invasive pneumococcal disease in children, particularly in disease caused by penicillin-resistant strains. The incidence of pneumococcal disease is likely to fall in Australia but there remains concern about the possibility of a rise in disease caused by non-vaccine serotypes (‘serotype replacement’). Despite the decreasing incidence of pneumococcal disease, this bacterium remains the most common cause of pneumonia, otitis media and meningitis in children.
Most human disease caused by 23 serotypes (of the 90 serotypes that have been described). 7 serotypes (those in the conjugate vaccine) cause about 85% of invasive disease in non-Aboriginal Australian children. Incidence of invasive disease is about 50–100 cases per 100 000 children aged <5 years. Incidence in central Australian Aboriginal children is the highest in the world (∼1000–2000 per 100,000 aged <5 years, before the introduction of pneumococcal vaccine). Antibiotic resistance is becoming a problem worldwide – organisms are classified as penicillin susceptible, penicillin-non-susceptible (PNSP), and cefotaxime/ ceftriaxone susceptible or resistant. Resistance may be intermediate or high-level.
Clinical features
Pneumonia and otitis media are the most common presentations, followed by septicaemia and meningitis. Soft tissue, bone and joint infections are less common manifestations.
Diagnosis
Meningitis, septicaemia or other sterile site infection is confirmed by culture/Gram stain of appropriate specimen (blood, CSF, joint fluid, peritoneal fluid, etc.). Pneumococcal pneumonia is blood culture positive in only about 10–20% of cases. PCR-based testing of blood and CSF is available and useful when antibiotics have been initiated before collection of specimens. Pneumococcal urinary antigen has poor specificity in children due to frequent pharyngeal carriage of pneumococcus.
Treatment
Penicillin or amoxicillin is the drug of choice, except in CNS infection with PNSP.
- If non-CNS infection with PNSP, treat with high-dose penicillin (benzylpenicillin 60 mg/kg (max. 2 g) i.v. 4 hourly for invasive disease including pneumonia, or amoxicillin 90– 120 mg/kg per day p.o. divided into three or four doses for otitis media or sinusitis).
- If CNS infection with cefotaxime/ceftriaxone-non-susceptible pneumococci, use vancomycin plus rifampicin or, for PNSP with cefotaxime MIC ≤ 2.0 mcg/mL, use high-dose cefotaxime (300 mg/kg per day) as an alternative (see Bacterial meningitis, p. 407). Duration of treatment is 10 days for meningitis and usually 5–7 days for other infections.
Vaccines
The 7-valent conjugate pneumococcal vaccine (7PCV, Prevenar®) has been part of the routine immunisation schedule in Australia since 2005 (2001 for indigenous children). 7PCV is given at 2, 4 and 6 months of age. A fourth dose in the second year of life is recommended for children at high risk of invasive pneumococcal disease, including those with hyposplenism. 9- and 13-valent conjugate vaccines are likely to be available in the near future.
The 23-valent polysaccharide vaccine provides protection against a broader range of serotypes but is not immunogenic in children <18–24 months of age, and protection is not long-lasting (2–3 years). The vaccine is routinely recommended in risk groups such as indigenous Australians, those with respiratory or cardiac co-morbidities, and patients with hyposplenia or certain immuno deficiencies.
Neisseria meningitidis
Neisseria meningitidis (meningococcus) is a Gram-negative diplococcus that mainly causes meningitis or septicaemia, or both. Less commonly, it may cause other infections including conjunctivitis, septic arthritis, pharyngitis, pneumonia, occult bacteraemia. For recommendations specific to meningitis see Bacterial meningitis, p. 407.
- Transmission: droplet.
- Incubation period: usually hours to 3 days.
- Infectious period: as long as carried – may be months. Most virulent within days of acquisition.
Epidemiology
- Peak age groups <2 years and 15–24 years.
- Serogroups B and C are most common in Australia (A, Y and W135 usually confined to travellers).
- Traditionally, group B is most common in young children and group C more common in adolescents.
- Incidence of group C meningococcal infection, which had been steadily rising in Victoria until 2003, has fallen rapidly since the introduction of routine conjugate group C vaccine.
- The case fatality rate has been reported as 5% for group B and 14% for group C infection.
Clinical features
- Meningitis – see below, p. 407.
- Septicaemia: often non-specific prodrome suggestive of viral illness.
- Rapid progression with any or all of the following: fever, rash (classically purpuric or petechial, but can be less specific), malaise, myalgia, arthralgia, vomiting, headache, reduced conscious state.
- Chronic meningococcaemia occurs rarely (consider terminal complement deficiency), and may be associated with progressive purpuric rash.
Diagnosis
Initially based on clinical features. Confirmation by culture of blood and/or CSF, preferably collected before first dose of antibiotics, although treatment should not be delayed. Additional tests may include shave biopsy of skin lesions (for Gram stain and culture), PCR on blood, CSF or skin sample.
Treatment
- Shock must be managed appropriately (see chapter 1, Medical emergencies).
- Discuss with Intensive Care for all cases of meningococcaemia and those with meningitis <2 years or very unwell.
- Immediate i.v. antibiotics (cefotaxime 50 mg/kg per dose (max. 2 g) 6 hourly, or if unavailable, benzylpenicillin 60 mg/kg per dose (max. 2 g) 4 hourly).
- Duration of antibiotics usually 7 days. Can change to i.v. benzylpenicillin when isolate identified as meningococcus. All cases should be notified immediately to statutory health authorities.
- Steroids
– Meningococcaemia: Consider corticosteroids (hydrocortisone 1 mg/kg i.v. 6 hourly) in severe cases.
– Meningitis: (Children > 4 weeks old) give dexamethasone 0.15 mg/kg i.v. 6 hourly.
- Immunoglobulin 0.5 g/kg i.v. over 4 hours, is considered in those requiring ICU.
Clearance antibiotics for contacts
Contacts >1 month old should receive prophylaxis (see Table 30.3, p. 412). Patients with invasive disease who have received only penicillin should also receive treatment to eradicate carriage.
Other aspects
Vaccines: Tetravalent polysaccharide vaccine (A, C, Y, W135) protects for a short time (3–5 years) and only in those aged >2 years. Further repeated vaccination is associated with development of tolerance to the vaccine. This vaccine is used for travellers (e.g. to African ‘meningitis belt’ or attending the Haj) and for controlling outbreaks. It is also used in patients with asplenia in conjunction with conjugate vaccine to provide broader protection. Meningococcal group C conjugate vaccine is effective in all age groups and given as part of the routine immunisation schedule to all Australian children at 12 months of age. Children at higher risk should be offered two doses of conjugate vaccine from 2 months of age with a third dose in the second year of life (see chapter 9, Immunisation).
Staphylococcus aureus
Staphylococcus aureus is a Gram-positive coccus that causes a wide variety of invasive and non-invasive disease.
Epidemiology
S. aureus is a common commensal, being present in the nose of about 1/3 of individuals. Both hospital- and community-acquired multi-resistant S. aureus (MRSA) are increasing problems.
Clinical features
Causes a variety of diseases including impetigo, boils and abscesses, cellulitis (including periorbital cellulitis), osteomyelitis, septic arthritis, endocarditis, pneumonia, food poisoning, bacteraemia, septicaemia and toxic shock syndrome. S.aureus is responsible for scalded skin syndrome in younger children. Staphylococcal infection may be accompanied by significant constitutional symptoms (e.g. myalgia) in addition to localising features.
Diagnosis
- Sterile site infection is confirmed by appropriate culture/Gram stain.
- Patients with S. aureus bacteraemia should undergo careful examination to exclude focal infection.
Treatment
- Surgical drainage is often necessary for abscesses and other foci of infection. Anti-staphylococcal antibiotics include flucloxacillin, cefalexin, cefazolin and clindamycin. MRSA is resistant to all penicillin and cephalosporin antibiotics.
- Community-acquired MRSA is often sensitive to a wide range of antibiotics including clindamycin, co-trimoxazole, ciprofloxacin, vancomycin, teicoplanin, rifampicin and fusidic acid. (Note: ciprofloxacin, rifampicin or fusidic acid should never be used as monotherapy for S. aureus as resistance develops rapidly.)
- Hospital-acquired MRSA is often only sensitive to vancomycin and teicoplanin. Although vancomycin may be necessary to treat some MRSA infections, it is not as effective as flucloxacillin for the treatment of susceptible patients.
- S. aureus (MSSA). Prolonged duration of treatment may be required to prevent recurrence.
Mycoplasma pneumoniae
- Transmission: droplet.
- Incubation period: 1–4 weeks.
- Infectious period: unknown, likely to be many months; typically infects all members of a family over a period of weeks/months although most are asymptomatic.
- Epidemiology: all ages (not just >5 years).
Clinical features
- Pneumonia: malaise, fever, headache, non-productive cough for 3–4 weeks (may become productive); 10% have rash (usually maculopapular); chest radiograph may demonstrate unilateral lobar or bilateral diffuse changes; bronchitis, pharyngitis, otitis media.
- CNS manifestations (uncommon; likely post-infectious): aseptic meningitis, meningoencephalitis, encephalitis, polyradiculitis/Guillain–Barré syndrome, acute cerebellar ataxia, cranial nerve neuropathy, transverse myelitis, acute disseminated encephalomyelitis and choreoathetosis.
Complications
Idiopathic thrombocytopenic purpura.
Diagnosis
- Serology: Not useful in acute setting. Infection can be diagnosed by fourfold rise in IgG over 2–4 weeks; IgM alone has very poor specificity in children as positive results occur in ∼30% of healthy preschoolers.
- Polymerase chain reaction (PCR) of respiratory specimens or CSF is now the gold standard but suffers from relatively poor sensitivity (sputum ∼70%, NPA ∼50%, throat swab ∼38%).
Treatment
- Roxithromycin 2.5–4 mg/kg (max. 150 mg) p.o. 12 hourly for 10 days is recommended, although its role is uncertain.
- Macrolides (e.g. azithromycin) are recommended in CNS disease although their role in this situation is also unclear.
Cytomegalovirus
Cytomegalovirus (CMV) is a ubiquitous herpes virus. It persists in latent form after primary infection and reactivation can occur years later, particularly with immunosuppression.
Transmission
- Horizontal: salivary contamination or sexual transmission; blood transfusion/organ transplantation.
- Vertical: transplacental, intrapartum via passage through infected genital tract and postnatal via ingestion of CMV-positive breast milk.
Incubation period
Unknown; infection usually manifests 3 weeks to 3 months after blood transfusion and 4 weeks to 4 months after tissue transplantation.
Clinical features
Vary with age and immunocompetence of child; asymptomatic infection is most common. Cytomegalovirus mononucleosis: cervical lymphadenopathy; hepatosplenomegaly in children, fever in adults. Note, clinical signs of CMV infection are similar to graft rejection in transplant patients. Both events peak 30–90 days after transplantation.
Diagnosis
Identification of the virus by culture or PCR of urine or saliva in the first 3 weeks of life is diagnostic of congenital disease; after this period, positive results may represent postnatally acquired infection. PCR of stored dried blood spots taken for the neonatal screening test may aid the diagnosis.
Distinguishing past from active infection can be difficult. Viral culture (rapid enhanced tissue culture immunofluorescence) or PCR of urine, saliva and even blood may be misleading as CMV can be excreted intermittently for life after primary infection, especially in immunocompromised patients. Leucocyte antigenaemia assay (degree of antigenaemia) correlates with the severity of CMV disease and is therefore a good means of predicting disease and monitoring progression in immunocompromised patients.
Complications
- Encephalitis, myocarditis, pneumonia, haemolytic anaemia, thrombocytopenia are rare manifestations.
- Primary CMV infection has been described in conjunction with Guillain–Barré syndrome and other peripheral neuropathies.
- Pneumonia, retinitis, hepatitis and colitis in immunocompromised.
- Congenital infection: >90% appear normal at birth: CNS sequelae in 10–20% of these (mainly sensorineural hearing loss); 5% present early with petechiae, hepatosplenomegaly, microcephaly and thrombocytopenia and have high rates of neurological sequelae.
Treatment
- Ganciclovir or valacyclovir for active CMV disease in the immunocompromised. Cytomegalovirus hyperimmune globulin is also sometimes used in these patients.
- Ganciclovir therapy should be considered in any neonate with symptomatic congenital CMV to reduce the risk of hearing loss and other neurological sequelae.
Enterovirus (non-polio)
Coxsackie A, B and echoviruses are important causes of childhood infections, especially in the summer months. These include a wide range of clinical presentations, including nonspecific febrile illness; pharyngitis; herpangina; hand, foot and mouth disease; gastroenteritis; aseptic meningitis; encephalitis; myocarditis; pericarditis and several forms of viral exanthem (maculopapular, vesicular, petechial). Infection in agammaglobulinaemic patients can cause particularly severe or persistent meningoencephalitis. In neonates, enteroviral infection may be difficult to distinguish clinically from bacterial sepsis.
Hand, foot and mouth disease
- Cause: coxsackie A virus (usually A16).
- Transmission: direct contact/droplet.
- Incubation period: 3–6 days.
- Infectious period: until blisters have gone.
Clinical features
Vesicles on cheeks, gums, sides of the tongue; papulovesicular lesions of palms, fingers, toes, soles, buttocks, genitals, limbs (may look haemorrhagic); sore throat; fever and anorexia.
Diagnosis
Tests are usually unnecessary as the clinical picture is sufficient for diagnosis.
Control of case
Exclusion is unnecessary (as virus is excreted in faeces for weeks).
Treatment
Symptomatic.
Epstein–Barr virus (infectious mononucleosis)
- Incubation period: 30–50 days.
- Infectious period: unknown, viral excretion from oropharynx for months.
Clinical features
Glandular fever: Fever, malaise, exudative tonsillopharyngitis, generalised lymphadenopathy and hepatosplenomegaly. Highly variable clinical course: acute phase lasts 2–4 weeks and convalescence may take weeks to months. May be associated with hepatitis or CNS involvement. In immunocompromised (particularly transplant patients), can cause severe lymphoproliferative disease.
Diagnosis
Atypical lymphocytes in the peripheral blood. Monospot test in blood for heterophile antibody identifies 90% of cases in older children and adults, but lacks sensitivity in children under 4–5 years of age. Serology is the gold standard. PCR of blood or tissue may be helpful in transplant patients.
Complications
Upper airway obstruction; dehydration from poor oral intake (uncommon). An unknown minority may develop symptoms of chronic fatigue syndrome. See also chapter 15, Adolescent health.
Treatment
Symptomatic: prednisolone 1 mg/kg (max. 50 mg) oral, daily may be considered in patients hospitalised for airway obstruction. Amoxicillin and ampicillin cause florid rash in up to 90% of children with EBV infection.
In patients with splenomegaly, avoidance of contact sports is recommended for 3–4 weeks after the onset of the illness to prevent splenic rupture.
Herpes simplex virus (HSV)
Manifestations of HSV infection include skin and mucous membrane involvement, gingivostomatitis (mainly HSV-1), genital herpes (mainly HSV-2), eczema herpeticum (see chapter 23, Dermatologic conditions), herpetic whitlow and eye involvement. HSV encephalitis is an important treatable condition that must not be missed (see p. 413 and chapter 33, Neurologic conditions, p. 461). Pneumonia and disseminated infection occur in the immunocompromised. Congenital infection also occurs. Infection can be primary (e.g. gingivostomatitis) or from a reactivation of the latent virus (e.g.cold sores).
Primary herpes gingivostomatitis
- Transmission: droplet, direct contact.
- Incubation period: 2–14 days
- Infectious period: indeterminate; virus can be excreted for at least 1 week, occasionally months. Shed intermittently with or without symptoms (including cold sores) for years afterwards.
Clinical features
Fever, irritability, cervical lymphadenopathy, halitosis, diffuse erythema and ulceration within the oral cavity (buccal mucosa, palate, gingiva and tongue) and mucocutaneous junction. Duration 7–14 days.
Diagnosis
Immunofluorescence or culture of vesicular scrapings.
Complications
Poor oral intake; autoinoculation resulting in herpetic whitlow, keratitis or genital herpes; eczema herpeticum; dissemination (particularly in immunocompromised).
Treatment
Symptomatic: topical anaesthesia (e.g. 1–2% lignocaine [lidocaine] gel, xylocaine viscous), analgesia (paracetamol), fluids and a soft diet. Aciclovir, valaciclovir or famciclovir should be considered if immunocompromised.
HSV in pregnancy
Primary infection during the first 20 weeks of gestation is associated with an increased risk of spontaneous abortion, stillbirth and congenital disease. Beyond 20 weeks, premature labour and growth retardation are more common. Primary infection after 34 weeks is associated with high rates of neonatal disease.
Neonatal HSV
Transmission
- Intrapartum (70–85%): perinatal acquisition from maternal genital tract; usually presents day 5–19.
- Postnatal (10%): usually presents day 5–19.
- Intrauterine (5%): transplacental; usually presents within 48 h of birth.
to infants with neonatal HSV infection give no history of genital HSV in themselves or their partners. The risk of infection to a baby of an asymptomatic woman with a history of recurrent genital herpes is <3%.
Clinical features
Neonatal infection presents in three ways:
- Localised skin, eye and/or mouth (‘SEM’) disease (45%). Onset 7–14 days. Death is rare; 30% or more of patients eventually develop evidence of neurological impairment.
- CNS disease (50%). Onset 14–21 days, in the form of encephalitis or a more disseminated disease. Mortality is 15%; 50–60% of survivors have psychomotor retardation, with or without microcephaly, spasticity, blindness, etc.
- Disseminated disease (20%). Onset 5–10 days. Involves any organ but primarily liver and adrenals; encephalitis occurs in 70% or more of patients. Presentation includes irritability, seizures, respiratory distress, jaundice, coagulopathy, shock and characteristic vesicular rash. Note: About 20% of babies never have skin lesions. Mortality is 50–60% (in spite of treatment) and neurological sequelae in 40%.
Diagnosis
Viral isolation from neonatal vesicular fluid, mouth or conjunctival swabs, stool, urine, leucocytes and maternal genital tract swabs. HSV antigens are detected by immunofluorescence. Serology is not always helpful, as maternally acquired IgG confounds interpretation in the neonate and IgM may not be produced until 2 weeks after the onset of illness. Detection of viral DNA by PCR (especially in CSF to detect subclinical CNS disease) is helpful. Changes on EEG, CT and MRI may all provide supporting evidence of HSV infection.
Complications
Overall mortality (following treatment) is 15–20% and 40–50% of infants have some neurological impairment.
Treatment
Aciclovir 20 mg/kg i.v. 8 hourly (see Antimicrobial guidelines, Appendix 3) for at least 14 days (SEM disease) or 21 days (CNS or disseminated disease). Suppressive treatment with aciclovir, for 6–12 months, should be considered for CNS or disseminated disease.
Prevention
20% of newborns born to women with primary HSV will be infected, even if delivered by Caesarean section.
HSV encephalitis
See Encephalitis, p. 413 and chapter 33, Neurologic conditions, p. 461.
Human herpes virus 6 (roseola infantum)
95% of children are infected with human herpes virus 6 (HHV-6) by the age of 2 years. Up to 30% will present with the clinical features of roseola. HHV-7 has also been shown to be the cause in a small number of children. HHV-6 infection may also present as an acute febrile illness without a rash.
- Transmission: direct contact/droplet (asymptomatically shed).
- Incubation period: 9–10 days.
- Infectious period: unknown (greatest during period of the rash).
Clinical features
Roseola: fever with occipital lymphadenopathy; then rapid defervescence corresponding with appearance of a red, maculopapular rash over trunk and arms lasting 1–2 days.
Note: Many children are started on antibiotics for the fever and then misdiagnosed as having a drug reaction when the rash appears.
Diagnosis
Investigations do not usually alter management, but serology and PCR are available.
Complications
Febrile convulsions (HHV-6 is thought to be the cause of up to 1/3 of febrile convulsions in children <2 years of age), aseptic meningitis, encephalitis (rare), hepatitis.
Treatment
Symptomatic.
Influenza virus
- Cause: influenza A or B virus.
- Transmission: direct contact/droplet.
- Incubation period: 1–4 days
- Infectious period: 3–7 days after onset of symptoms. Longer in immunocompromised.
Epidemiology
Continuous genetic re-assortment of influenza A viruses can lead to epidemics; the degree of cross-immunity from previously circulating strains and vaccines determines whether this occurs. Large-scale epidemics (pandemics) occur when there is no protection from previous exposure. Although the virus may cause disease at any time, seasonal epidemics occur during the winter months.
Clinical features
Variable. Severity of illness is dependent on partial immunity from previous exposure to related influenza viruses and vaccines. Asymptomatic infection occurs. Commonly presents with fever and rigors; respiratory symptoms including coryza, pharyngitis, cough, pneumonia, wheeze or croup; headache, myalgias, fatigue. Vomiting and diarrhoea are less common.
Complications
Important complications include bacterial superinfection with pneumonia (especially S. aureus), otitis media or sinusitis; neurological: encephalitis, meningitis, encephalopathy; myositis and cardiomyopathy. Death occurs in ∼1% of hospitalised children.
Diagnosis
- Rapid diagnosis by immunofluorescence or PCR on respiratory specimens (nasal swab or NPA) may lead to early treatment.
- Viral culture is also important for epidemiology.
Control of case
Exclude from school until resolution of symptoms.
Treatment
- Neuraminidase inhibitors (zanamavir, oseltamivir) reduce the severity and length of illness by up to 36 h if started within 48 h, but earlier initiation is associated with the best outcome. Routine treatment of influenza in immunocompetent patients is not recommended.
- Neuraminidase inhibitors (oseltamivir ≥1 year of age, zanamavir ≥7 years of age) should be considered in children with laboratory-confirmed influenza, who are immunocompromised or who have chronic medical conditions.
Prevention
Vaccines can be used to prevent influenza in children.
- Trivalent inactivated intramuscular vaccine is ∼65% protective against influenza and prevents ∼30% of influenza-like illnesses, but efficacy varies from year to year. Annual vaccination with vaccine containing the most recent strains is necessary to provide continued protection. Two doses at least one month apart are recommended for children <9 years who are receiving influenza vaccine for the first time.
- Live attenuated vaccine is more effective (∼79% protective) but is not yet available in Australia.
Neuraminidase inhibitors may be used to prevent influenza in children at risk of complications.
Measles virus (rubeola)
As a result of widespread measles immunisation, this disease is now seen infrequently. However, outbreaks continue to occur in most parts of the world.
- Transmission: droplet, direct contact.
- Incubation period: 7–18 days (usually 14 days) to the appearance of a rash.
- Infectious period: 1–2 days before the onset of symptoms to 4 days after the onset of the rash.
Clinical features
- Prodrome: fever, conjunctivitis, coryza, cough and Koplik spots (white spots on a bright red buccal mucosa).
- Rash: appears 3–4 days later; erythematous and blotchy; starts at hairline and moves down the body, then becomes confluent; lasts 4–7 days; may desquamate in the second week.
Diagnosis
Serology (IgM is usually detectable 1–2 days after onset of rash, and almost always 4 days after), immunofluorescence and culture or PCR on nasopharyngeal aspirate.
Complications
Otitis media (1/4), pneumonia (1/25), encephalitis (1/2000), subacute sclerosing panencephalitis (SSPE) (1/25 000).
Treatment
Symptomatic: vitamin A should be considered for young infants with severe measles, the immunocompromised and those with vitamin A deficiency.
Control of case
Exclude from school for at least 5 days from the appearance of the rash.
Contacts
- Measles, mumps, rubella (MMR) vaccine should be given within 72 h of exposure, to unimmunised children >9 months of age (another dose should be given at 12 months of age or 4 weeks after the first dose, whichever is the later).
- If MMR is contraindicated, or if >72 h since exposure, normal human immunoglobulin should be given i.m. within 7 days (see chapter 29, Haematologic conditions and oncology).
- Exclude from school for 2 weeks if unimmunised.
Parvovirus B19 (erythema infectiosum, slapped cheek disease, fifth disease)
- Transmission: droplet, direct contact.
- Incubation period: 4–21 days.
- Infectious period: highly infectious until rash appears (50% of adults are immune).
Clinical features
Fever in 15–30%; non-specific prodrome. The rash has three stages:
- Slapped cheek appearance (1–3 days).
- Maculopapular rash: on proximal extensor surfaces, flexor surfaces and trunk; fades over next few days, then central clearing, forming a reticular pattern (after 7 days).
- Reticular rash: reappears with heat, cold and friction (weeks/months).
Diagnosis
Mainly clinical. PCR on blood and serology.
Arthritis; aplastic crisis in children with chronic haemolytic anaemia; bone marrow suppression; fetal hydrops.
Treatment
Symptomatic.
Control of case
School exclusion is inappropriate as the patient is no longer infectious once the rash appears.
Contacts
Pregnant contacts should seek advice regarding the unlikely possibility of intrauterine infection as treatment of fetal infection may prevent sequelae.
Rubella virus
- Transmission: droplet, direct contact.
- Incubation period: 14–21 days.
- Infectious period: 5 days before to 7 days after rash.
Clinical features
- 25–50% have no symptoms.
- Rash: small, fine, discrete pink maculopapules; starts on face and spreads to chest and upper arms, abdomen and thighs, all within 24 h.
- Prodrome: (1–5 days) low-grade fever, malaise, headache, coryza, conjunctivitis (more common in adults), postauricular/occipital/posterior triangle lymphadenopathy precedes rash by 5–10 days.
Diagnosis
Serology.
Complications
Congenital rubella syndrome: >25% affected if mother infected during 1st trimester; 10–20% have single congenital defect if infection occurs at 16–40 days.
Control of case
Exclude from school for at least 5 days from the onset of the rash.
Contacts
Check serology if pregnant. Immunoglobulin given after exposure in early pregnancy may not prevent infection or viraemia, but may modify risk of abnormalities in the baby.
Varicella zoster virus (chickenpox, shingles)
- Incubation period: 10–21 days. Shorter incubation in the immunocompromised. Zoster immune globulin (ZIG) may prolong incubation to 28 days.
- Infectious period: 1–2 days before appearance of the rash until the rash is fully crusted.
Clinical features
Fever, irritability, anorexia and lymphadenopathy. A pruritic rash develops over the next 3–5 days, which progresses from maculopapular to vesicular, followed by crusting by 5–10 days. Lesions appear in crops with a central distribution. Affects scalp, face, trunk, mouth, conjunctivae and extremities.
Diagnosis
Clinical diagnosis is usually sufficient. Immunofluorescence and culture or PCR of vesicular scrapings, or serology.
Complications
Secondary bacterial infection of skin lesions (most commonly group A streptococcus or S. aureus) or bacterial pneumonia; neurological (cerebellitis, transverse myelitis, Guillain–Barré syndrome); dissemination (pneumonitis, hepatitis, encephalitis) in patients with abnormal T-cell immunity. Herpes zoster (‘shingles’), resulting from a reactivation of the latent virus, is more common in children who have had chickenpox in infancy or who have been exposed in utero.
Treatment
- Aciclovir, famciclovir or valaciclovir in patients with impaired T-cell immunity. Antiviral treatment is not indicated in the immunocompetent child.
- Antibiotics for secondary bacterial skin infection (e.g. flucloxacillin).
- Aspirin is contraindicated because of the association with Reye’s syndrome.
- Other NSAIDs (including ibuprofen) should be avoided because of possible increased risk of invasive group A streptococcal disease.
- Paracetamol can be used.
Prevention
ZIG within 96 h of exposure (6 mL for adults, 4 mL for children 6–12 years of age, 2 mL for children up to 5 years of age). Indicated in the following patients in contact with varicella (or direct contact with shingles):
- Immunocompromised children (e.g. HIV, immunosuppressive therapy (including high-dose steroids; prednisolone 2 mg/kg or more per day) and patients with transplants, lymphoma, leukaemia or severe combined immune deficiency).
- Newborn infants whose mothers have varicella onset from within 7 days before delivery to 2 days after delivery.
- Infants under 28 days of age with non-immune mothers exposed to varicella.
- Hospitalised premature infants with no maternal history of varicella.
- Hospitalised premature infants <28 weeks gestation or <1000 g, regardless of maternal history.
Varicella vaccine
See chapter 9, Immunisation.
See also chapter 27, Gastrointestinal conditions.
Infectious diarrhoea continues to cause significant morbidity in children from developed and developing countries. In Australia, approximately 20 000 children (15/1000) <5 years of age are admitted to hospital each year with acute gastroenteritis. Rotavirus is the causal agent in up to 2/3 of hospitalised children where a pathogen is identified. Other important pathogens in hospitalised children include caliciviruses, enteric adenoviruses, astroviruses, Salmonella spp., Campylobacter jejuni, Giardia intestinalis (lamblia), Cryptosporidium parvum, enteropathogenic (and other) Escherichia coli, Shigella spp. and Yersinia enterocolitica.
- Children <5 years of age with rotavirus-positive gastroenteritis are unlikely to have another pathogen isolated from their faeces.
- It is unusual to find a protozoal parasite in the setting of acute diarrhoea.
- Repeat stool investigations are not helpful except in patients with chronic diarrhoea, suspected Salmonella carriage or parasitic infection.
- The cause of infectious diarrhoea can often be identified by simple laboratory studies but rarely alters management.
- Most bacterial causes of diarrhoea are self-limiting and do not usually require antibiotic therapy, even if blood or mucus is present. As with viral gastroenteritis, the primary aim of treatment is to achieve and maintain adequate hydration. Antibiotics should be considered for the immunocompromised and neonates.
- Nosocomial infection is common. Hence, adequate infection control measures established by the hospital are essential in preventing spread.
Rotavirus
- Incubation period: illness usually begins 12 h–4 days after exposure.
- Infectious period: most children shed the virus in the stools for up to 10 days; however, about 1/3 with severe primary rotavirus infection continue shedding for >21 days.
Clinical features
Major cause of severe diarrhoea in children causing over 50% of hospitalisations for acute gastroenteritis in children <5 years. Also a common cause of nosocomial infection. Annual peak period of infection occurs in the winter–spring period. Presents with diarrhoea, vomiting (may precede diarrhoea) and fever lasting for up to 1 week. Respiratory symptoms are common. May be complicated by dehydration, electrolyte imbalance and acidosis.
- Diagnosis: enzyme immunoassay and latex agglutination assay.
- Treatment: supportive, with particular attention to hydration.
- Vaccine: two safe and efficacious rotavirus vaccines are available. There is reasonable evidence that intussusception is not associated with these new vaccines (a concern with previous vaccines).
Adenovirus
Similar presentation to rotavirus, but there is no seasonality. It is more common under 12 months of age. Diarrhoea and vomiting may last longer and high fever is less common.
Salmonella (non typhi)
Clinical features
Broad spectrum of clinical syndromes including asymptomatic carriage, gastroenteritis, bacteraemia and focal infections (e.g. bone and joint). Age-specific attack rates are highest in children <5 years of age (peak at <1 year of age) and the elderly. Invasive infections and mortality are more common in infants, the elderly and those with underlying diseases.
Diagnosis
Does not usually alter management, but serology and PCR are available.
Treatment
Antibiotic treatment is not usually indicated for uncomplicated gastroenteritis as it may prolong excretion. Antibiotic treatment is indicated for bacteraemia, systemic involvement or infection in infants <3 months of age, those with underlying disease (e.g. immunocompromised) and elderly people. The choice and duration of treatment depends on the clinical manifestation and antibiotic susceptibility.
Campylobacter jejuni
More common >5 years of age. Causes diarrhoea with visible or occult blood, abdominal pain, malaise and fever. Antibiotic treatment is not usually necessary, except in special circumstances where the elimination of the carrier state is important, such as infection in food handlers.
Giardia intestinalis (lamblia)
Transmission
The most common parasite identified in stool specimens from children. More common in children (and staff) in childcare centres and returned travellers. The major reservoir and means of spread is contaminated water and, to a lesser extent, food. Person-to-person spread also occurs.
Clinical features
There is a broad spectrum of clinical manifestations, but the most common are: diarrhoea (usually persistent), abdominal distension, flatulence, abdominal cramps and weight loss/ failure to thrive.
Diagnosis
Confirmed by microscopy of stool specimens. These do not usually contain blood, mucus or leucocytes. Repeat specimens may be necessary.
Metronidazole 30 mg/kg (max. 2 g) p.o. daily for 3 days or tinidazole 50 mg/kg (max. 2 g) p.o. as a single dose are effective treatments for symptomatic giardiasis.
Dientamoeba fragilis
Transmission
This parasite is thought to be transmitted with the eggs of Enterobius vermicularis (pinworm).
Clinical features
Symptoms include acute or chronic diarrhoea and abdominal pain, although many infected children are asymptomatic. May be associated with eosinophilia.
Treatment
May be treated with metronidazole (dose as above) although treatment is unnecessary in asymptomatic patients where the organism is found incidentally.
Escherichia coli
There are at least five categories of diarrhoea-producing E. coli:
- Enterohaemorrhagic E. coli (EHEC): haemolytic uraemic syndrome (HUS), haemorrhagic colitis.
- Enteropathogenic E. coli (EPEC): watery diarrhoea in children <2 years of age in developing countries.
- Enterotoxigenic E. coli (ETEC): the major cause of traveller’s diarrhoea (usually self-limiting).
- Enteroinvasive E. coli (EIEC): usually watery diarrhoea, but may cause dysentery.
- Enteroaggregative E. coli (EAEC): chronic diarrhoea in infants and young children.
Antibiotic treatment is not usually indicated for diarrhoea caused by E. coli and may be associated with increased rates of HUS in EHEC infection.
Clostridium difficile
Transmission
Acquired from the environment or by faecal–oral transmission from a colonised host. Up to 50% of healthy neonates and infants <2 years of age are colonised, in contrast to 5% of those >2 years of age.
Clinical features
Rare cause of diarrhoea in those <12 months of age. Only clinically significant diarrhoea or colitis should be considered to be caused by Clostridium difficile. Pseudomembranous colitis usually occurs in patients on antibiotics (particularly penicillins, clindamycin and cephalosporins).
Treatment
- Cessation of antibiotics, and
- Oral metronidazole 7.5 mg/kg (max. 400 mg) p.o. 8 hourly for 10 days, and
- Consider probiotics – Saccharomyces spp. (baker’s or brewer’s yeast) or Lactobacillus spp.
Failure of treatment or recurrence may be due to reinfection, non-compliance, continued antibiotic use, or, rarely, a metronidazole-resistant organism. Options in this context include repeat metronidazole, oral vancomycin (not i.v.) or probiotics.
Enterobius vermicularis (threadworm, pinworm)
The most common worm infection in Australia. The highest rates of infection occurs in school age children, followed by preschoolers. In some groups, nearly 50% of children are infected.
Transmission
Eggs survive up to 2 weeks on clothing, bedding or other objects. Eggs often remain under the fingernails. Reinfection by autoinfection is common. Infection often occurs in more than one family member.
Incubation period
At least 1–2 months from the ingestion of eggs until the adult female migrates to the perianal region to deposit eggs.
Infectious period
Eggs are infective within a few hours of being deposited on the perianal skin.
Clinical features
Causes pruritus ani and vulvae.
Diagnosis
Visualisation of worms in the perianal region (at night) or microscopy of eggs collected on sticky tape briefly applied to perianal skin in the morning.
Treatment
Mebendazole 50 mg (<10 kg), 100 mg (>10 kg) p.o. (not in pregnancy or in those <6 months of age) or pyrantel 10 mg/kg (max. 750 mg) p.o. as a single dose, followed by a second dose 2 weeks later. All family members should be treated.
See also chapter 27, Gastrointestinal conditions.
Hepatitis A
Hepatitis A virus (HAV) is the most common viral hepatitis; it is particularly prevalent in developing countries.
- Transmission: faecal–oral route.
- Incubation period: usually about 4 weeks (2–7 weeks).
- Infectious period: viral shedding lasts 1–3 weeks; the highest titres in stool occur 1–2 weeks before the onset of illness, corresponding to the highest risk of transmission; lowest risk after onset of jaundice.
Clinical features
Usually an acute self-limited illness; mild, non-specific symptoms without jaundice in infants and preschoolers; fever, malaise, jaundice, anorexia and nausea in older children and adults.
Complications
Relapse (unusual), fulminant hepatitis (rare).
Diagnosis
Serology for HAV-specific IgM and IgG.
Treatment
Supportive.
Control of case
Children should be excluded from childcare or school for 7 days from the onset of illness, although the virus is excreted for many weeks.
Prevention
Inactivated HAV vaccine is recommended for travellers to endemic areas and patients with chronic liver disease (e.g. hepatitis B or C infection) or transfusion dependent illness.
Hepatitis B
Hepatitis B virus (HBV) infection is endemic worldwide. The prevalence of HBV and carriage rates vary in different parts of the world. The carriage rate is about 0.2% in Australians of European origin, and >10% in some indigenous populations. Rates are highest in those born in Asian or Mediterranean countries.
Transmission
Blood or body fluids that are HBsAg positive; vertical transmission occurs in infants born to HBsAg-positive mothers; there is a high risk of horizontal transmission in the first 5 years of life.
- Incubation period: 7 weeks–6 months.
- Infectious period: from several weeks before the onset until documented clearance of virus.
Clinical features
Symptomatic acute hepatitis (jaundice, anorexia, malaise and nausea) in adults; usually asymptomatic in young children, particularly in those <1 year of age.
Complications
The carrier state is associated with 25% mortality from hepatocellular carcinoma or chronic liver disease; lifelong follow-up is indicated. Those infected as infants or young children are more likely to become carriers and develop fatal complications as adults: 70–90% of infants infected at birth become chronic HBV carriers (particularly if the mother is HBeAg positive), in contrast to only 5% of adults. The remainder eliminate the virus and have no long-term effects.
Diagnosis
Test for:
- HBsAg (active disease).
- HBsAb (protection by vaccine or natural infection).
- HBeAg or HBV PCR (increased infectivity and risk of sequelae).
- HBcAb (past or present HBV infection).
Treatment
No specific therapy for HBV is available; á-interferon and nucleoside analogues may resolve chronic infection but are less effective if infection is acquired during childhood. Hepatitis A vaccination is recommended.
Prevention
- In Australia, recombinant HBV vaccine is currently recommended for all infants from birth, pre-adolescents, as well as those at high risk. Infants born to HBsAg-positive mothers should be given HBV-specific immunoglobulin plus a full course of HBV vaccination. They should be tested at 12–18 months with HBsAg and HBsAb.
- See needle-stick injuries (p. 419) for management of exposure to infected blood or body fluid.
Hepatitis C
Hepatitis C virus (HCV) causes acute and chronic hepatitis. The carriage rate is about 0.3% in apparently healthy new blood donors in Australia, but this probably underestimates the prevalence in the population, which may be around 1%.
Transmission
Parenteral exposure to HCV-infected blood and blood products; vertical transmission occurs from about 6% of HCV-positive mothers (higher if the mother is co-infected with HIV). Avoid use of fetal scalp electrodes; breast-feeding is not contraindicated but temporary avoidance is recommended if bleeding or cracked nipples; sexual transmission is rare.
- Incubation period: 6–7 weeks (range 2 weeks–6 months).
- Clinical features: mild, insidious hepatitis; usually asymptomatic in children.
- Complications: persistent infection in >85% (most children with chronic infection are asymptomatic); 65–70% develop chronic hepatitis, 20% develop cirrhosis. Uncommonly, hepatocellular carcinoma can develop in the absence of chronic hepatitis.
- Diagnosis: current or past infection is detected by serology (anti-HCV antibodies); current infection is confirmed by PCR for HCV RNA.
- Treatment:
– Patients must be screened for chronic hepatitis, cirrhosis and hepatocellular carcinoma.
– Optimal treatment regimens are under investigation. Hepatitis A and B vaccination is recommended.
Hepatitis E
Hepatitis E virus (HEV) is an uncommon cause of hepatitis, which occurs predominantly in tropical countries, especially in parts of India. Cases have been reported in travellers returning from these regions.
- Transmission: faecal–oral route, by contamination of water.
- Incubation period: 2–10 weeks.
- Infectious period: excreted in stool for 2 weeks after the onset of symptoms.
- Clinical features:
– Similar to HAV infection. Most infected children have asymptomatic infection or mild gastrointestinal symptoms.
– Mortality is rare, except in pregnant women.
- Diagnosis:
– HEV-specific IgM.
– PCR of stool and serum.
- Prevention: food and water safety in endemic countries.
Bacterial meningitis is a medical emergency.
Clinical features
- In infants, non-specific e.g. fever, lethargy, irritability or vomiting.
- In older children, headache, vomiting, drowsiness, photophobia and neck stiffness may be present. Kernig sign (inability to extend the knee when the leg is flexed at the hip) and Brudzinski sign (bending the head forward produces flexion of the legs) may be positive.
Diagnosis
Diagnosis is confirmed by examination of the cerebrospinal fluid (CSF), unless lumbar puncture (LP) is contraindicated (see chapter 3, Procedures). If LP is deferred or reveals no organism, identification of the pathogen may still be possible through:
- Blood cultures – often positive.
- PCR on blood or CSF for enterovirus, HSV and other viruses, TB, N. meningitidis and S. pneumoniae.
- Blood smear for Gram stain.
- Skin scraping or aspirate of purpuric lesions for N. meningitidis on Gram stain, PCR or (less likely) culture.
- Throat swab.
Antibiotics must be given immediately after the collection of appropriate cultures, but should not be delayed if the LP is to be deferred. Antibiotics should be rationalised to more specific treatment based only on final CSF or blood culture results.
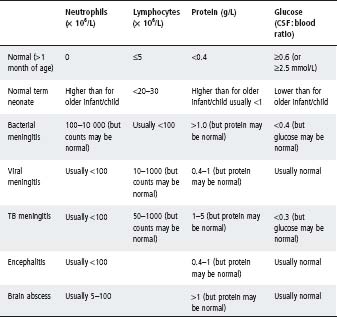
Interpretation of cerebrospinal fliuid findings
CSF findings should always be interpreted in the light of the clinical setting (see Table 30.2).
Cell count
- Perform microscopy without delay. Cell lysis begins shortly after collection: neutrophils may decrease by up to 1/3 after 1 h and by 1/2 after 3 h (lymphocytes may decrease by ∼10% after 2 h).
- Macroscopic appearance of CSF may be misleading: 200–500 × 106/L cells are required before CSF appears cloudy to the naked eye.
- In early bacterial meningitis there may be no increase in the CSF cell count.
- CSF contaminated by blood can be difficult to interpret. There is no reliable rule to correct for red blood cells (RBC) in CSF, although a ratio of one white blood cell to 500–700 red blood cells is sometimes used. Similarly, 0.01 g/L protein for every 1000 RBCs may be allowable. It is safer to be cautious and interpret the CSF as if it has not been contaminated with blood.
- Even in bacterial meningitis, the CSF cell count may remain normal in up to 4% of young infants and up to 17% of neonates.
- Presence of neutrophils in the CSF should always raise concern (except in neonates, see Table 30.2).
- In early viral (typically enteroviral) meningitis, the CSF findings can mimic bacterial meningitis with a neutrophil predominance. This shifts to a lymphocytic picture after 6–8 h.
- In bacterial meningitis there can be a shift to a lymphocyte predominance after 48 h of therapy.
- Listeria infection is associated with a lower neutrophil rise than other causes of bacterial meningitis.
- Gram stain may be negative in up to 60% of cases of bacterial meningitis even without prior antibiotics.
- Antibiotics usually prevent the culture of bacteria from the CSF, but they do not significantly alter the CSF cell count nor biochemistry in samples taken early. In ‘partially treated meningitis’ the CSF should be interpreted like any other CSF.
- Seizures do not cause an increased CSF cell count.
- In neonates, interpretation of CSF may be difficult. Normal values for CSF cell counts and biochemistry differ from those of older infants (typically they have higher cell count and protein and lower glucose, particularly in premature neonates) (see Table 30.2).
Biochemistry
- CSF protein is normal in about 40% of school-age children with bacterial meningitis.
- CSF glucose is normal in about 1/2 of school-age children with bacterial meningitis.
- CSF glucose may be decreased in mumps meningitis and lymphocytic choriomeningitis, as well as in bacterial and TB meningitis.
Table 30.2 Classical cerebrospinal fluid (CSF) findings
Adjunctive steroid treatment of meningitis
- There is now sufficient evidence to recommend the routine use of adjunctive steroid therapy for bacterial meningitis in children >4 weeks old, as this can reduce the risk of hearing loss.
- Steroids are recommended at the time of LP where there is a strong clinical suspicion of meningitis.
- Antibiotics should not be delayed for >30 min to enable administration of steroids.
– Initial dose: dexamethasone 0.15 mg/kg i.v. ideally given 15 min before, but up to 1 h after, the first dose of antibiotics.
– Ongoing dose: dexamethasone 0.15 mg/kg i.v. 6 hourly should be continued for 4 days (unless bacterial meningitis has been excluded).
Antibiotic treatment of meningitis
Age >2 months
The incidence of bacterial meningitis has fallen dramatically since the introduction of conjugated Haemophilus influenzae type b (Hib) vaccine. The major pathogens are now Streptococcus pneumoniae and Neisseria meningitidis.
Penicillin (and cephalosporin)-resistant pneumococci (PRP) are an increasing problem worldwide. Local patterns of resistance dictate treatment.
- Initial therapy: cefotaxime 50 mg/kg (max. 2 g) i.v. 6 hourly.
- In areas with a significantly high incidence of PRP, or when PRP are suspected, vancomycin 15 mg/kg (max. 500 mg) i.v. 6 hourly should be added to a third generation cephalosporin as empiric therapy.
- Continued therapy: antibiotic treatment is adjusted according to culture and sensitivity results to complete (i.v. therapy):
– 7 days for N. meningitidis.
– 10 days for S. pneumoniae.
– 7–10 days for Hib.
- If there is prolonged or secondary fever, or where sensitivity testing indicates the pneumococcal isolate has reduced susceptibility to third-generation cephalosporins, LP should be repeated to detect treatment failure, and CT brain should be considered, looking for abscess or empyema formation.
- For sensitive S. pneumoniae or N. meningitidis: benzylpenicillin 60 mg/kg (max. 2 g) i.v. 4 hourly, or amoxicillin 50 mg/kg (max. 2 g) i.v. 4 hourly, or continue with cefotaxime.
Age <2 months
The organisms responsible for meningitis in this age group can be either neonatal pathogens (e.g. group B streptococcus (GBS), Escherichia coli and other enteric Gram negatives, and Listeria monocytogenes), or those more commonly detected in older children (e.g. Streptococcus pneumoniae, Neisseria meningitidis, Hib).
- Initial therapy: i.v. benzylpenicillin, cefotaxime and gentamicin (see Antimicrobial guidelines, Appendix 3).
- Continued therapy: treatment is adjusted according to culture and sensitivity results. Gentamicin is used for its synergistic action with penicillin for the treatment of GBS and Listeria meningitis. Therapy should be continued for:
– 2–3 weeks in GBS and Listeria meningitis
– At least 3 weeks in Gram-negative coliform meningitis.
Meningitis associated with shunts, neurosurgery, head trauma and CSF leak
In addition to the organisms discussed above, meningitis in these circumstances can be caused by Staphylococcus aureus, coagulase negative staphylococci and (with a ventriculoperitoneal shunt in situ) Gram-negative bacilli including Pseudomonas aeruginosa.
- Initial therapy: vancomycin 15 mg/kg (max. 500 mg) i.v. 6 hourly plus:
– (with ventriculo-peritoneal shunt) ceftazidime 50 mg/kg (max. 2 g) i.v. 8 hourly
– (without ventriculo-peritoneal shunt) cefotaxime, 50 mg/kg (max. 2 g) i.v. 6 hourly
Antibiotic prophylaxis for contacts of meningitis cases
See Table 30.3.
General measures
Requirement for intensive care
Admission to ICU should be discussed with a specialist in the following circumstances:
- Age <2 years.
- Coma.
- Cardiovascular compromise.
- Intractable seizures.
- Hyponatraemia.
Fluid management
Careful fluid management is important in the treatment of meningitis as many children develop the syndrome of ‘inappropriately’ increased antidiuretic hormone secretion (SIADH). The degree of fluid restriction varies with each patient according to their clinical state. Hypovolaemia should be corrected with 10 mL/kg of normal saline repeated as required. A patient who is not in shock and has a normal serum sodium should usually be given 50% maintenance fluid requirements as initial management. If the serum sodium is <135 mmol/L give 25–50% of maintenance requirements. Serum sodium should be measured every 6–12 h for the first 48 h and the total fluid intake adjusted accordingly.
Observations
Neurological observations and blood pressure should be done every 15 min for the first 2 h and then at intervals determined by the child’s conscious state. Head circumference should be monitored daily. Weight is measured daily or more frequently if required.
Seizures
Hypoglycaemia, electrolyte imbalance (especially hyponatraemia) and raised intracranial pressure should be excluded before attributing seizures to the underlying infection or febrile convulsion. Control of seizures is vital and specialist consultation is advised.
Analgesia
Ensure adequate analgesia; children in the recovery phase may have significant headache.
Fever persisting for >7 days
May be due to nosocomial infection, subdural effusion or other foci of suppuration. Uncommon causes include inadequately treated meningitis, a parameningeal focus or drugs.
Outcome/follow-up
All patients require a hearing assessment 6–8 weeks after discharge, or sooner if hearing loss is suspected.
More than 1/4 survivors have mild disabilities that adversely affect school performance and behaviour. Consequently, all children surviving bacterial meningitis should be regularly reviewed during their early school years. Less common sequelae include epilepsy, visual impairment and cerebral palsy.
Table 30.3 Prophylaxis regimens for contacts of meningitis cases
| Organism | Antibiotic | Those requiring prophylaxis |
| Haemophilus influenzae type b | Rifampicin 20 mg/kg (max 600 mg) p.o. daily for 4 days Infants <1 month of age: Rifampicin 10 mg/kg p.o. daily for 4 days Pregnancy/contraindication to rifampicin: Ceftriaxone 250 mg i.m. daily for 2 days | • Index case and all household contacts if household includes other children <4 years of age who are not fully immunised. |
| • Index case and all household contacts in households with any infants <12 months of age, regardless of immunisation status. | ||
| • Index case and all household contacts in households with a child 1–5 years of age who is inadequately immunised. | ||
| • Index case and all room contacts, including staff, in a childcare group if index case attends >18 h/week and any contacts <2 years of age who are inadequately immunised. | ||
| • AND children who are not up to date with Hib should be immunised. | ||
| Neisseria meningitidis | Rifampicin 10 mg/kg (max 600 mg) p.o. 12-hourly for 2 days Infants <1 month of age: Rifampicin 5 mg/kg p.o. 12-hourly for 2 days Pregnancy/contraindication to rifampicin: Ceftriaxone 250 mg (>12 y) or 125 mg (<12 y) i.m. as a single dose or Ciprofloxacin 500 mg (>12 y) or 250 mg (6–11 y) p.o. as a single dose | • Index case (if treated only with penicillin) and all intimate household or day care contacts who have been exposed to index case within 10 days of onset. |
| • Any person who gave mouth-to-mouth resuscitation to the index case. | ||
| Streptococcus pneumoniae | Nil | • No increased risk to contacts. |
Notes:
• It is important that rifampicin is given early to both the index case and contacts, especially for N. meningitidis disease, because of the rapidity with which secondary cases may develop.
• As prophylaxis is not infallible, any febrile household contact should seek urgent medical attention.
• Nasopharyngeal carriage of Hib is not eradicated by a single injection of ceftriaxone.
• Rifampicin interferes with the metabolism of several medications, including the oral contraceptive pill (alternative contraception should be instituted), anticonvulsants, warfarin and chloramphenicol.
• Rifampicin colours body fl uids red, e.g. urine, saliva, tears (soft contact lenses may be damaged), sweat, etc.
Prevention
Many cases of meningitis are now preventable. All parents should be encouraged to have their children fully immunised (see chapter 9, Immunisation).
Viral meningitis
The most common causes of viral meningitis or meningoencephalitis are enteroviruses (Coxsackie and echoviruses) and HHV-6 (see p. 396). Most cases are self-limiting; however, their clinical presentation can mimic bacterial meningitis. Enterovirus may be isolated from throat swabs and stools, and PCR of the CSF may be positive. Treatment is symptomatic except in the rare instance of infection in the immunocompromised where i.v. immunoglobulin (IVIG) may be used. Positive enteroviral PCR on CSF in a child with a consistent clinical presentation can allow for early cessation of antibiotics and discharge from hospital.
Tuberculous meningitis
Tuberculous meningitis is uncommon in Australia. It often presents in an insidious manner and can be difficult to recognise. Large volumes of CSF are required (at least 10 mL) for diagnosis by microscopy and culture of mycobacteria, or mycobacterial PCR (which is not necessarily more sensitive). Treatment with combination antitubercular antibiotics plus corticosteroids should be started early and requires specialist advice.
Encephalitis
Encephalitis is most commonly caused by HSV-1 or 2, EBV, VZV, enterovirus, adenovirus, influenza virus or Mycoplasma pneumoniae. Encephalitis usually presents with one or more of the following: fever, headache, vomiting, change of behaviour, drowsiness, convulsions (particularly focal), focal neurological deficits and signs of raised intracranial pressure. CSF findings are non-specific (see Table 30.2). CT or MRI of the brain and EEG may be more helpful.
The recognition of herpes encephalitis is critical because treatment with aciclovir is indicated. Focal seizures and neurological signs are more typical of herpes encephalitis but clinical presentation, especially early in the disease, is not specific to a particular pathogen. Therefore, any child with encephalitis of an uncertain cause should be started on i.v. aciclovir (see Antimicrobial guidelines, Appendix 3). If the patient does not regain consciousness over a short period of time, i.v. aciclovir should be continued until:
- An alternative diagnosis is reached; or
- Herpes encephalitis is excluded by:
– Absence of typical clinical features.
– Normal serial MRI scans.
– Normal serial EEG.
– Negative PCR for HSV on CSF obtained >72 h into the illness (this may necessitate a repeat LP).
Macrolides (e.g. azithromycin) are sometimes used in encephalitis due to M. pneumoniae but their benefit is uncertain.
Brain abscess
Brain abscess classically presents with fever, headache and focal neurological deficit. Although it is rare, early recognition is important because most cases are readily treated and delayed diagnosis can be disastrous. Diagnosis is by brain CT or MRI. Empiric treatment to cover the major aetiological pathogens is flucloxacillin 50 mg/kg (max. 2 g) i.v. 4 hourly, cefotaxime 50 mg/kg (max. 2 g) i.v. 6 hourly and metronidazole 15 mg/kg (max. 1 g) i.v. stat, then 7.5 mg/kg (max. 500 mg) i.v. 8 hourly (see Antimicrobial guidelines). Aspiration for diagnosis or neurosurgical intervention is usually necessary.
Kawasaki disease (KD) is a systemic vasculitis that predominantly affects children under 5 years of age. Although the specific causal agent remains unknown, it is believed that KD is initiated by an infectious agent, although it is not transmitted from person to person. Early recognition and treatment are essential to reduce the risk of life-threatening complications.
Diagnosis
Diagnosis is often delayed because the features are similar to those of many viral exanthems. The diagnostic criteria for KD are:
- Fever for 5 days or more; plus
- Four of the following five features:
– Polymorphous rash.
– Bilateral (non-purulent) conjunctivitis.
– Mucous membrane changes; e.g. reddened or dry cracked lips, strawberry tongue, or a diffuse redness of oral or pharyngeal mucosa.
– Peripheral changes; e.g. erythema of the palms or soles, oedema of the hands or feet and desquamation in convalescence, particularly involving skin of hands, feet or perineal region.
– Cervical lymphadenopathy (>15 mm in diameter, usually unilateral, single, non-purulent and painful).
- Exclusion of diseases with a similar presentation: staphylococcal infection (e.g. scalded skin syndrome and toxic shock syndrome), streptococcal infection (e.g. scarlet fever and toxic shock-like syndrome, but not just isolation from throat), measles, adenovirus and other viral exanthems, leptospirosis, rickettsial disease, Stevens–Johnson syndrome, drug reaction and juvenile chronic arthritis.
Particular clinical vigilance is needed to recognise patients with ‘incomplete’ or ‘atypical’ KD. Such patients do not fulfill the formal diagnostic criteria, but are still at risk of developing coronary artery aneurysms. Other relatively common features include arthritis, diarrhoea and vomiting, coryza and cough, and hydropic gallbladder.
Investigations
Laboratory features may include neutrophilia, raised erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), mild normochromic, normocytic anaemia, raised transaminases, hypoalbuminaemia and marked thrombocytosis in the second week.
Complications
Up to 30% of untreated children develop coronary artery dilation or aneurysm formation. This can occur up to 6–8 weeks after the onset of the illness. Echocardiography should therefore be done at least twice: at presentation and, if negative, again at 6–8 weeks to exclude coronary artery involvement. Coronary artery aneurysms can be associated with severe early ischaemic heart disease.
Management
Management includes early (preferably within the first 10 days of the illness) administration of IVIG (single dose of 2 g/kg i.v. over 10 h) and aspirin (3–5 mg/kg p.o. once a day (anti-platelet dose) for at least 6–8 weeks; maximum dose 150 mg). There is no evidence that using high (anti-inflammatory) dose aspirin decreases the risk of aneurysm development over and above that prevented by IVIG. However, some guidelines suggest using high dose aspirin (10 mg/kg p.o. 8 hourly) until defervescence. Paracetamol can be used for symptomatic relief.
A repeat dose of IVIG (2 g/kg) should be given to patients who fail to defervesce within 24 h after the completion of the first dose. Refractory cases with continuing fever and other signs of inflammation subsequent to this need specialist advice for further treatment that may include high-dose steroids (e.g. methylprednisolone 30 mg/kg i.v.).
Treatment with IVIG is highly effective in preventing the potentially devastating complication of coronary artery involvement. Treatment should still be undertaken in patients presenting after 10 days of illness if they have evidence of ongoing inflammation (fever, raised acute phase markers).
This is usually caused by an infection or inflammation of the lymph nodes. Malignancy is much less common.
Infectious causes
Acute bilateral lymphadenitis
- Viral upper respiratory tract infections.
- Systemic viral infections (e.g. EBV and CMV: may have generalised lymphadenopathy and hepatosplenomegaly).
- Kawasaki disease: may present initially as cervical lymphadenitis alone (see p. 414 above).
Acute unilateral lymphadenitis
- Group A streptococcus or Staphylococcus aureus: 40–80% of acute unilateral lymphadenitis; occurs at 1–4 years of age; fever, tenderness, overlying erythema; may be associated with cellulitis.
- Anaerobic bacteria: older children with dental caries or periodontal disease.
- Group B streptococcus may have overlying cellulitis (neonates).
Subacute/chronic unilateral lymphadenitis
- Bartonella henselae (cat-scratch disease): occurs about 2 weeks after a scratch or lick from a kitten or dog, usually involves axillary nodes, tender nodes; there may be a papule at infection site.
- Mycobacterium avium complex (MAC – formerly known as MAIS): patient usually 1–4 years of age, afebrile, systemically well and not immunocompromised; node usually unilateral, slightly fluctuant, non-tender, sometimes tethered to underlying structures and with violaceous hue to overlying skin.
- Toxoplasma gondii: systemic features (fatigue, myalgia), there may be generalised lymphadenopathy.
- Mycobacterium tuberculosis: usually a contact history; affects older children; systemic symptoms (e.g. fever, malaise, weight loss), non-tender nodes.
- HIV.
Management
Acute bilateral lymphadenitis without other signs (e.g. pallor, bruising or hepatosplenomegaly) is usually of viral cause and needs no specific treatment or investigation. Acute unilateral lymphadenitis with a fluctuant node needs incision and drainage (contra-indicated in suspected TB as may result in sinus formation). Otherwise, acute unilateral lymphadenitis is treated with oral flucloxacillin 25 mg/kg (max. 500 mg) p.o. 6 hourly for 10 days (see Antimicrobial guidelines, Appendix 3), with review in 48 h.
Clinical features
- An infection of cutaneous and subcutaneous tissue characterised by erythema, warmth, oedema and tenderness.
- Predisposing factors include a break in the skin (e.g. insect bite, trauma) or a pre-existing skin lesion.
- May be associated with regional lymphadenopathy, fever, chills and malaise.
- It may be associated with deeper involvement including necrotising fasciitis, osteo myelitis and septic arthritis.
- Usually caused by Streptococcus pyogenes or Staphylococcus aureus.
- Haemophilus influenzae type b (Hib) is uncommon but should be considered in non-immunised children <5 years. It is often accompanied by bacteraemia or meningitis, or both.
Diagnosis
Cultures of blood, skin aspirate or skin biopsy are positive in about 25% of cases.
Management
- Flucloxacillin 25 mg/kg (max. 500 mg) p.o. 6 hourly.
- Parenteral therapy is needed if there is fever, rapid progression, lymphangitis or lymphadenitis. Non-immunised children <5 years with facial cellulitis should be treated with cefotaxime 50 mg/kg (max. 2 g) i.v. 6 hourly, and flucloxacillin.
Gram-positive bacteria (group A â-haemolytic streptococci and Staphylococcus aureus) can cause disease as a result of production of protein (superantigen) toxins.
Clinical features
Fever, erythematous rash, conjunctivitis, reddened mucous membranes, strawberry tongue and prolonged capillary refill time. A range of clinical presentations may be seen. At the most severe end of the spectrum, capillary leak leads to hypotension, shock and multi-organ failure (toxic shock syndrome).
This condition may also be associated with the use of tampons and specific information should be sought.
Diagnosis
Early diagnosis depends on recognition of clinical features. Culture results may help to confirm the diagnosis later.
Management
A critical part of early management is to remove any possible focus of infection (including retained tampon if appropriate).
Early recognition of shock with appropriate fluid management and intensive care is important. Antibiotics should include an anti-staphylococcal agent (e.g. flucloxacillin 50 mg/ kg (max. 2 g) i.v. 4 h, see Antimicrobial guidelines). Treatment should also include clindamycin (to inhibit bacterial toxin and host cytokine production) and i.v. immunoglobulin (as an immunomodulatory agent).
Cause
Acquired immunodeficiency syndrome (AIDS) is caused by human immunodeficiency virus (HIV).
Transmission
Perinatal (vertical) transmission is the most common mechanism of paediatric HIV infection.
Incubation period
It is important to distinguish between infection with HIV, which may be asymptomatic during a variable latent period, and the progressive immunological derangement that leads to AIDS. Perinatally infected infants may be asymptomatic for several months or years.
Risk groups
- Infants of mothers who are known to be HIV positive or who are members of a high-risk group (e.g. sex workers, i.v. drug users and those with bisexual partners).
- I.v. drug users.
- Men who have sex with men.
- Sexual contacts (including sexually abused children) of individuals with HIV.
- Transfusion recipients, particularly patients with congenital bleeding disorders who received blood products before 1985.
- Individuals from countries with a high prevalence of HIV infection.
Clinical features
Clinical presentations of HIV infection include prolonged fever, failure to thrive or weight loss, generalised lymphadenopathy, hepatosplenomegaly, parotitis, chronic or recurrent diarrhoea, recurrent otitis, chronic candidiasis and chronic eczematous rash.
The indicator diseases for the diagnosis of AIDS in children include candidiasis, lymphoid interstitial pneumonitis, recurrent episodes of serious bacterial infection, opportunistic infection (e.g. Pneumocystis carinii pneumonia and disseminated Mycobacterium avium complex disease), CMV retinitis, cerebral toxoplasmosis, progressive neurological disease and malignancy (e.g. primary brain lymphoma).
Diagnosis
Patients (± their families) require counselling and informed consent before testing for HIV, which should be done on a confidential basis. Specific antibody detection is a sensitive indicator of HIV infection in adults and children, but passively transferred maternal antibodies may persist for up to 18 months in infants. Prior to 18 months, ultra sensitive HIV PCR is the best available test to confirm the diagnosis of HIV infection. The disease is monitored using a combination of CD4+ T-cell count and quantification of HIV RNA (viral load). Patients may also have lymphopaenia, abnormal T-cell subsets and hypergammaglobulinaemia.
Management
A multidisciplinary approach by a specialised team is vital for the unique needs of these patients and their families. Medical management of HIV-positive patients includes:
- Antiretroviral drugs (highly active antiretroviral treatment, HAART).
- Prevention of opportunistic and other infections (immunisation and prophylactic antimicrobials).
- The early diagnosis and aggressive management of opportunistic infections.
Control
Antiretroviral therapy given to the HIV-infected woman during pregnancy and delivery, and to the newborn, complete avoidance of breast-feeding (where formula is available and safe) and with other measures can decrease the rate of transmission to the child from 25–35% to <2%. Recognition of HIV-infected pregnant women is therefore critical.
Antiretroviral therapy and other interventions (e.g. immunisation, Pneumocystis prophylaxis) can have a significant impact on disease progression.
Community acquired needle-stick injuries
The risk of sero conversion to HIV, HBV or HCV from a community-acquired needle-stick injury is very low. Exposed individuals should be reassured. Immunity to hepatitis B should be confirmed, and if incomplete, hepatitis B vaccine should be given. Unless the injury is considered to be particularly high risk, no further management is required at the time. Follow-up should be arranged for counselling and serology if required. Tetanus toxoid ± immunoglobulin should be considered.
Occupational needle-stick injuries
Standard precautions
- All sharp objects and body fluids should be considered as potentially contaminated.
- Avoid contact with blood and other body fluids by:
– Using protective barriers (e.g. gloves) if contact is likely.
– Immediately cleaning up accidental spills.
Managing needle-stick injury or exposure to blood/blood-stained body fluid
- Squeeze the puncture wound.
- Wash blood off the skin with soap and water.
- Rinse blood from the eyes and mouth with running water.
- Document the date and time of exposure, details of incident, names of the source and exposed individuals.
- Inform source individual of exposure.
- Assess the risk of HIV, HBV and HCV in the source individual (see below).
- If indicated, test known source for HBV surface antigen (HBsAg), HCV antibody (anti-HCV Ab) and antibodies to HIV-1 and HIV-2 (HIV Abs). Obtain consent from the source individual.
- Even if the source individual is not infected with a blood-borne pathogen, storage of a serum specimen from the exposed person is recommended.
- Follow-up should be arranged for counselling (of the exposed person).
- IVDU past or current (particularly if they share equipment)
- Give HBV-specific immunoglobulin ± HBV vaccine if appropriate (see Tables 30.4, 30.5).
- HIV post-exposure prophylaxis is only required if source is HIV Ab positive, or if the source is unknown and HIV is considered likely.
Table 30.4 Evaluation of needle-stick injury sources
| High risk of HIV and HBV | High risk of HCV |
| Unsafe sex, particularly with multiple (or homosexual) partners | Recipients of blood products prior to 1985 |
| Intravenous drug users (IVDU) (particularly if they share equipment) and their sexual partners | IVDU past or current (particularly if they share equipment) |
| Family members of an infected person | |
| Individuals from communities with high HIV prevalence |
Table 30.5 Management of needle-stick injury
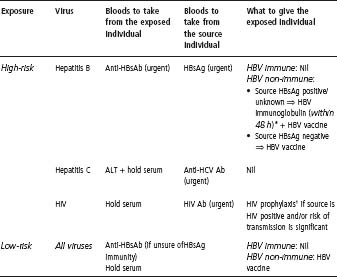
* HBV immunoglobulin should be given as soon as possible, but can be deferred for 48 h, while awaiting results of serology to confi rm affected individual’s immunity (when checking whether a vaccinated individual has maintained immunity or whether the individual is immune from previous infection).
† Urgent expert advice should be sought.
Assessing risk
A significant exposure is considered to have occurred if there has been:
- An injection of blood/body fluid (particularly if >1 mL).
- A skin-penetrating injury with a sharp that is contaminated with blood/body fluid.
- A laceration from a contaminated instrument.
- A direct inoculation in the laboratory with contaminated material.
- A contaminated wound or skin lesion.
- Mucous membrane/conjunctival contact with blood/body fluid.
The incidence of HBV, HCV and HIV in Victorian i.v. drug users is 1.8, 10.7 and 0.2 per 100 person–years, respectively. The estimated risk of virus transmission from an occupational needle-stick injury from a known positive donor (e.g. in Victoria) is:
- HBV: 6–30%
- HCV: 0–7%
- HIV: ∼0.4%.
Note: These figures are for needle-stick injury from a positive source. When the source is unknown, the actual risk of infection for the affected individual depends on the probability of infection in the source population.
USEFUL RESOURCES
- http://www.health.vic.gov.au/ideas/bluebook/ – Australian national information on infectious diseases and their control, from the Department of Human Services. Has detailed information on most infections.
- http://www.cdc.gov – The US Centers for Disease Control and Prevention has an enormous array of information on infectious diseases and travel medicine.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


